on ABIONYX (EPA:ABNX)
ABIONYX Pharma Receives FDA Approval for New Sepsis Study
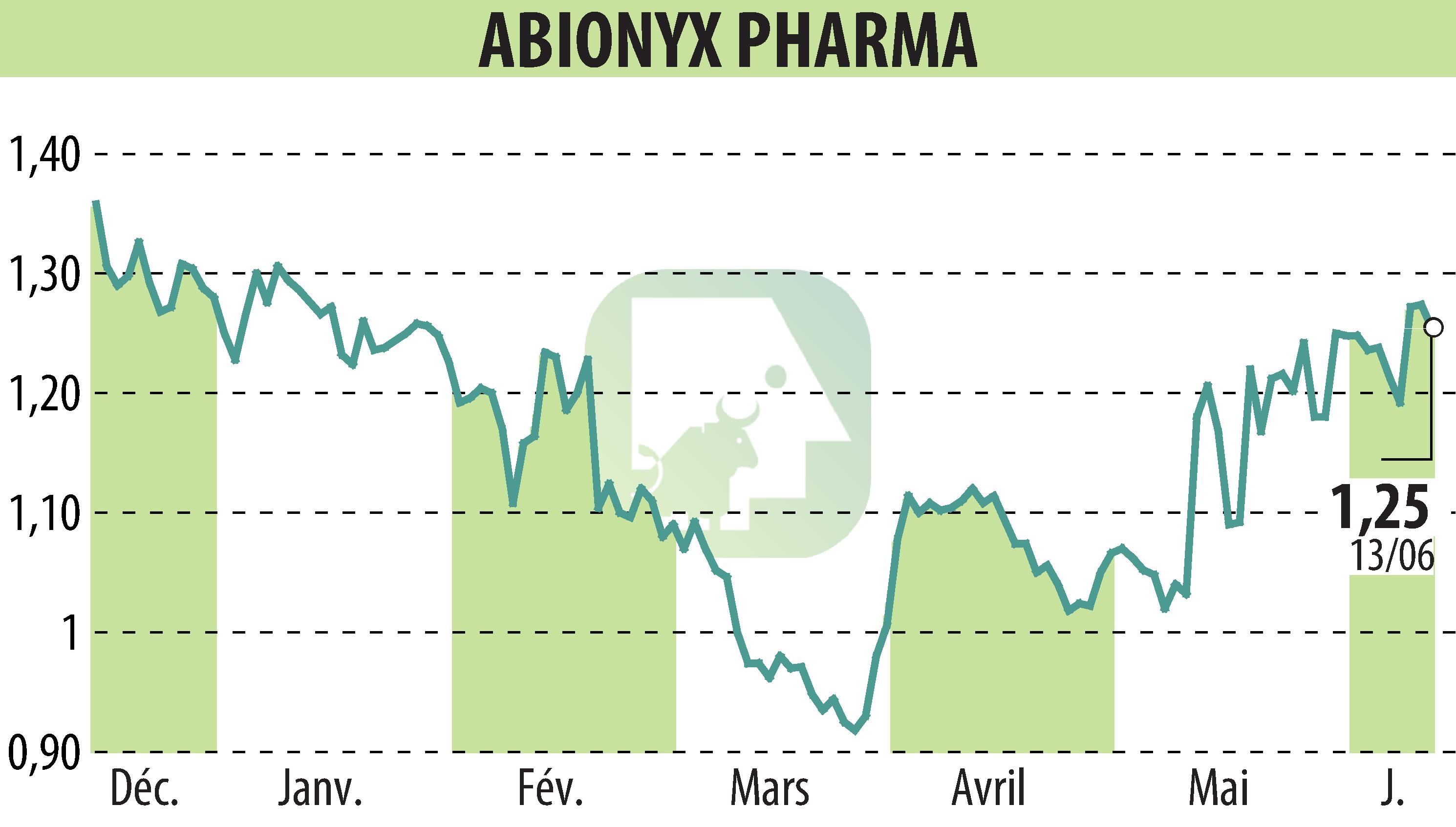
ABIONYX Pharma announces that it has successfully completed the pre-IND meeting with the FDA for the initiation of a phase 2b/3 clinical study targeting sepsis. The company will file its IND application in the coming months.
The success is based on promising phase 2a results published in BMC Medicine. Studies have shown a significant reduction in endotoxins, inflammatory cytokines and endothelial markers in patients with sepsis, thanks to treatment with CER-001, a recombinant human apolipoprotein AI.
Dr. Rob Scott, Chief Medical Officer of ABIONYX, highlights the importance of this milestone for the development of CER-001, hoping to significantly improve the treatment of sepsis in the future.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABIONYX news
