on ABSCIENCES (EPA:AB)
AB Science: Green light for phase 3 study of masitinib in prostate cancer
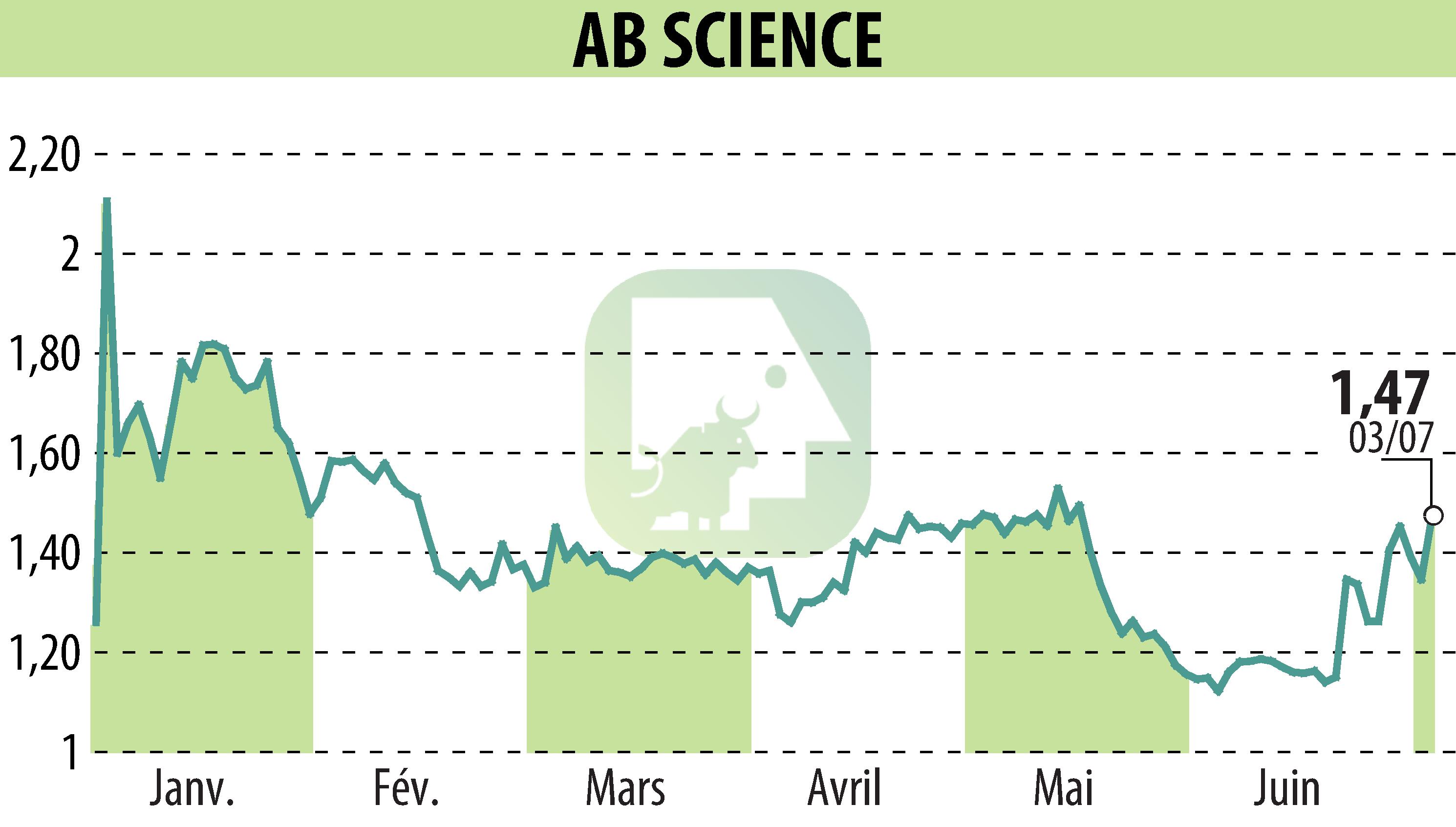
AB Science announced FDA and EMA approval for a Phase 3 study of masitinib, targeting hormone-refractory metastatic prostate cancer (mCRPC). This study, named AB22007, will use masitinib in combination with docetaxel for patients with a specific biomarker, promising a potential advance in the treatment of mCRPC. With 600 patients enrolled, the study will evaluate radiographic progression-free survival.
Masitinib is positioned in combination with docetaxel, addressing an unmet medical need in mCRPC. Past results indicate that the alkaline phosphatase biomarker can predict treatment response, marking significant therapeutic potential. Intellectual property protection is secured through 2042 via granted European patents, with additional applications pending in the United States.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABSCIENCES news
