on Aspire Biopharma, Inc. (NASDAQ:PWUP)
Aspire Biopharma Receives IRB Approval for Rapid-Acting Aspirin Trial
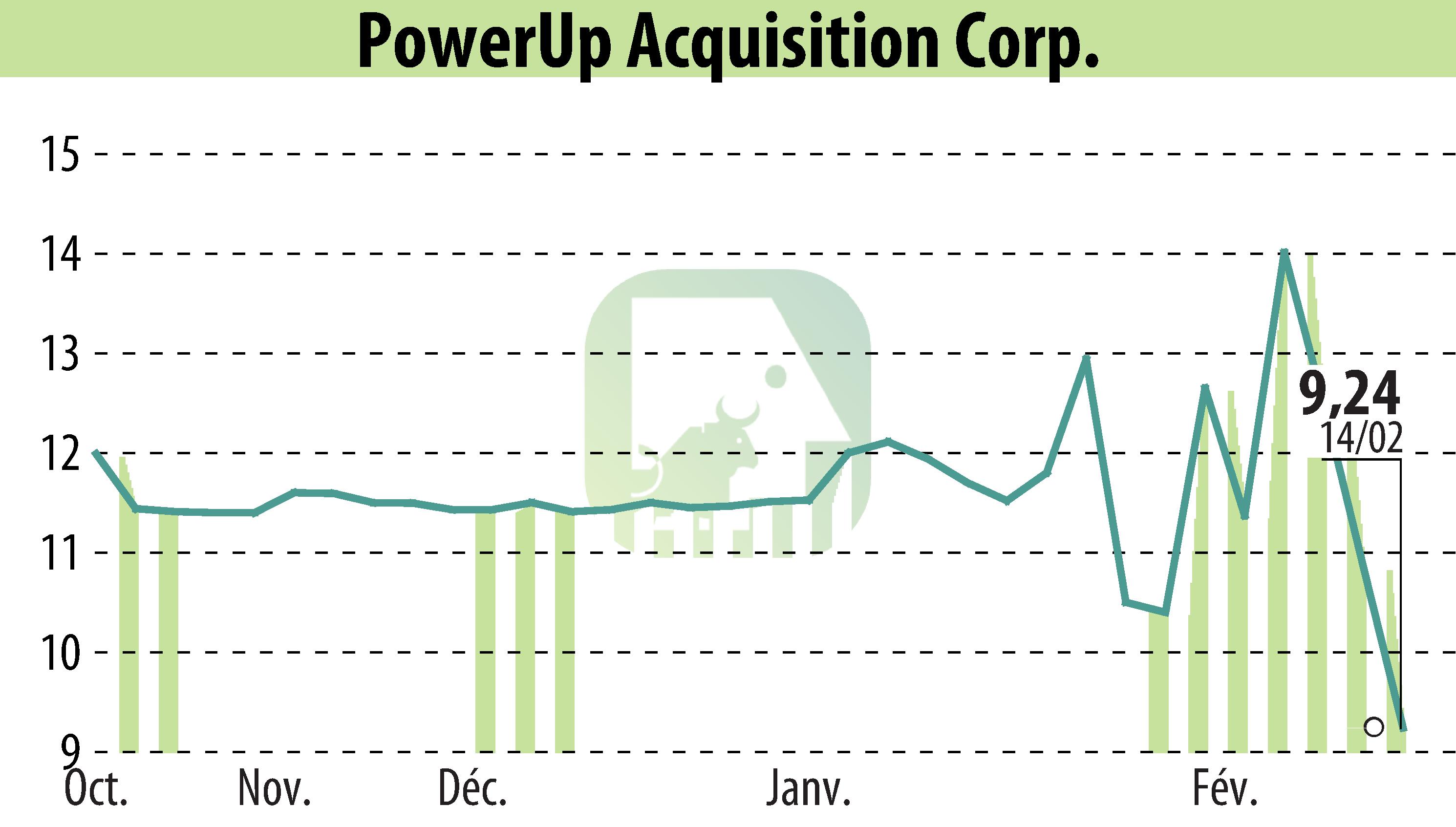
Aspire Biopharma Holdings, Inc. has announced Institutional Review Board (IRB) approval for its upcoming Phase I clinical trial in the U.S. This trial will evaluate safety, pharmacokinetics, and pharmacodynamics of a new oral transmucosal fast-acting high-dose aspirin formulation. Enrollment is set for 6-8 participants with trials beginning imminently.
The trial will compare pharmacokinetic and pharmacodynamic data between volunteers given sublingual aspirin powder and those receiving standard oral aspirin. This study will inform future trials and potential FDA fast-track approval for treating suspected acute myocardial infarction.
The sublingual formulation is designed for rapid absorption, aiming to improve treatment outcomes, especially in emergency cardiac situations. Greater bioavailability may also enhance aspirin's effects while reducing gastric irritation risks.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Aspire Biopharma, Inc. news
