on Bausch Health Companies Inc. (NASDAQ:BHC)
Bausch Health Announces Updates on Norwich XIFAXAN Litigation and New FDA Submission
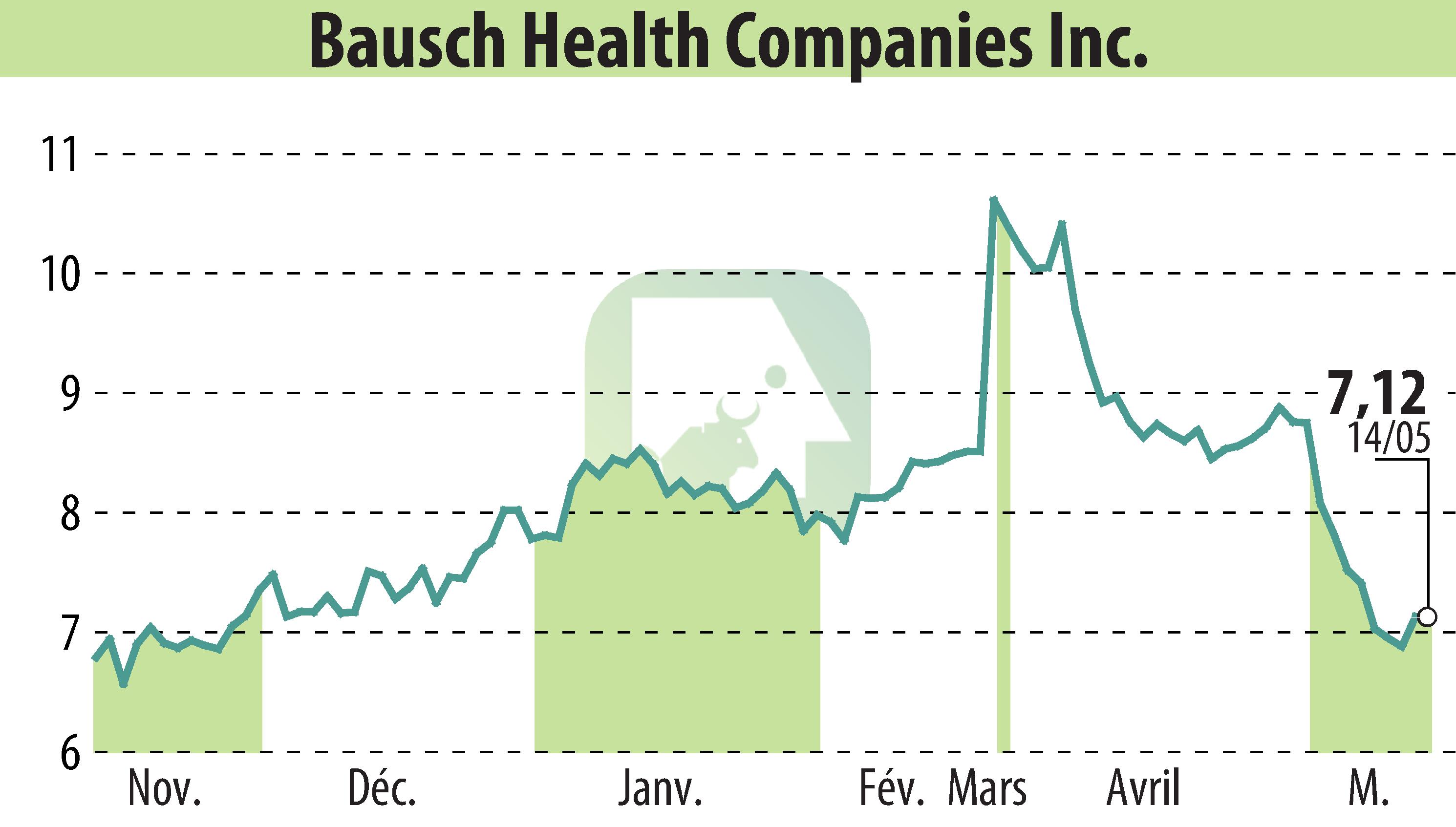
Bausch Health Companies Inc., along with Salix Pharmaceuticals, provided an update concerning ongoing litigation with Norwich Pharmaceuticals and a new FDA submission by Norwich. The update follows the recent decision by the US Court of Appeals for the Federal Circuit on April 11, 2024. Both parties have filed petitions for a rehearing which is expected to be concluded within the next three months.
Norwich has informed Bausch Health of its submission to the FDA, seeking approval to market a generic version of XIFAXAN® 550 mg. Bausch Health has a 45-day window from the date of this notice to initiate a patent infringement lawsuit, which would delay FDA approval of the generic by up to 30 months or until a court decision. Bausch Health confirmed its commitment to vigorously defend its intellectual property rights for XIFAXAN®.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Bausch Health Companies Inc. news
