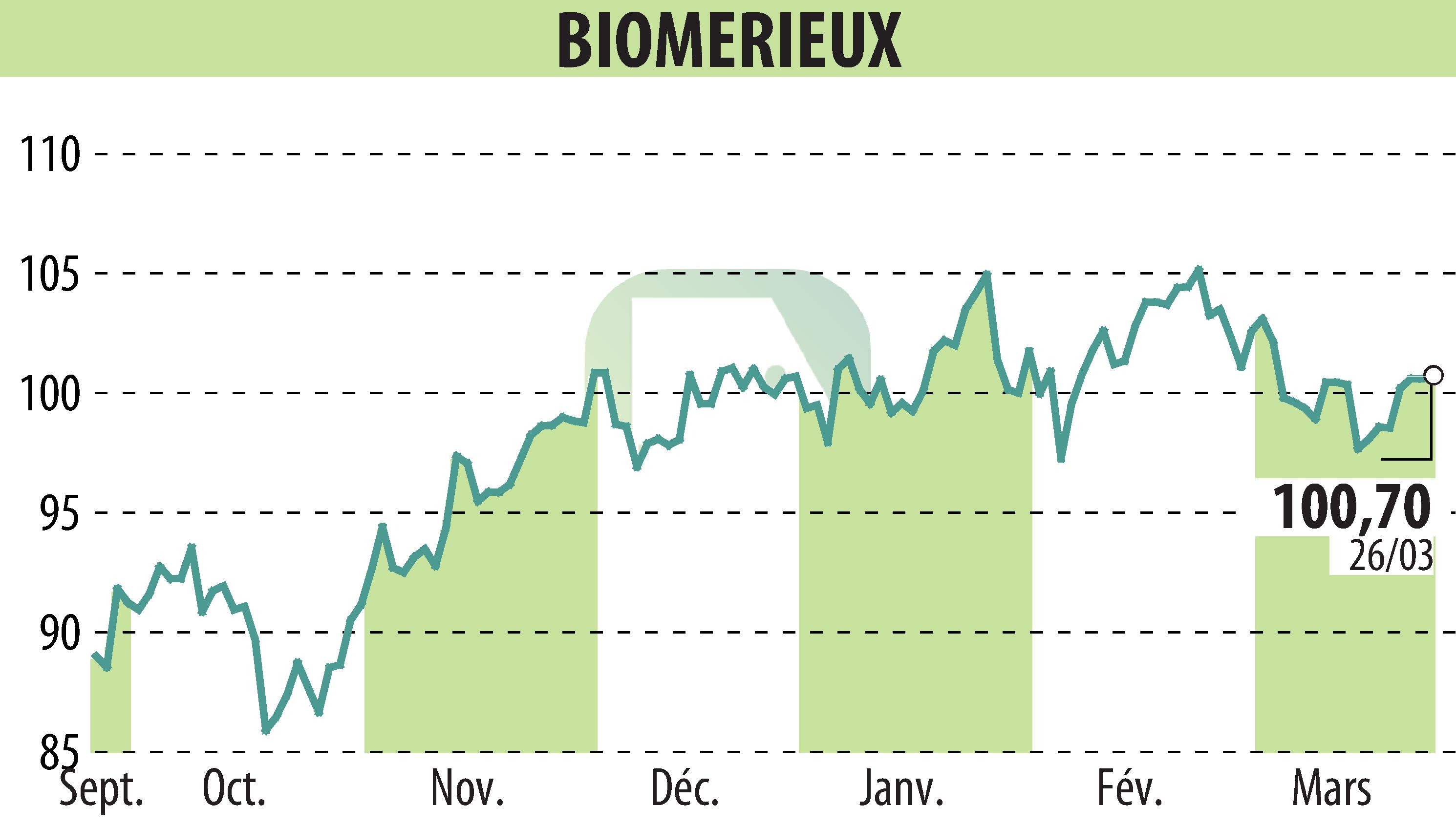
on BIOMERIEUX (EPA:BIM)
BioMérieux Obtains 510(k) Clearance and CLIA Waiver for BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel
New rapid and innovative diagnostic panel for respiratory and pharyngeal infections
By Regis PIERPONT - BioMérieux has obtained 510(k) clearance and CLIA waiver from the US Food and Drug Administration (FDA) for the BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) panel. This unique multiplex PCR test can detect and identify in approximately 15 minutes the nucleic acids of 15 bacteria, viruses and viral subtypes most commonly responsible for respiratory or pharyngeal infections.
Healthcare professionals can now access rapid and innovative diagnostic tests, such as the BIOFIRE® SPOTFIRE® R/ST panel, to meet the new medical needs generated by the COVID-19 pandemic. The flexibility of this syndromic panel allows clinicians to screen for multiple pathogens whose signs and symptoms are identical for patients, allowing informed decisions to be made during the outpatient consultation.
The BIOFIRE® SPOTFIRE® R/ST Panel is the third panel cleared by the FDA on the BIOFIRE® SPOTFIRE® System, with two other panels available: the BIOFIRE® SPOTFIRE® Respiratory (R) Panel and the BIOFIRE® SPOTFIRE® Respiratory (R) ) Panel Mini. Thanks to the CLIA exemption, the BIOFIRE® SPOTFIRE® system and the relevant panels can be used by people who are not laboratory professionals and in all healthcare settings.

