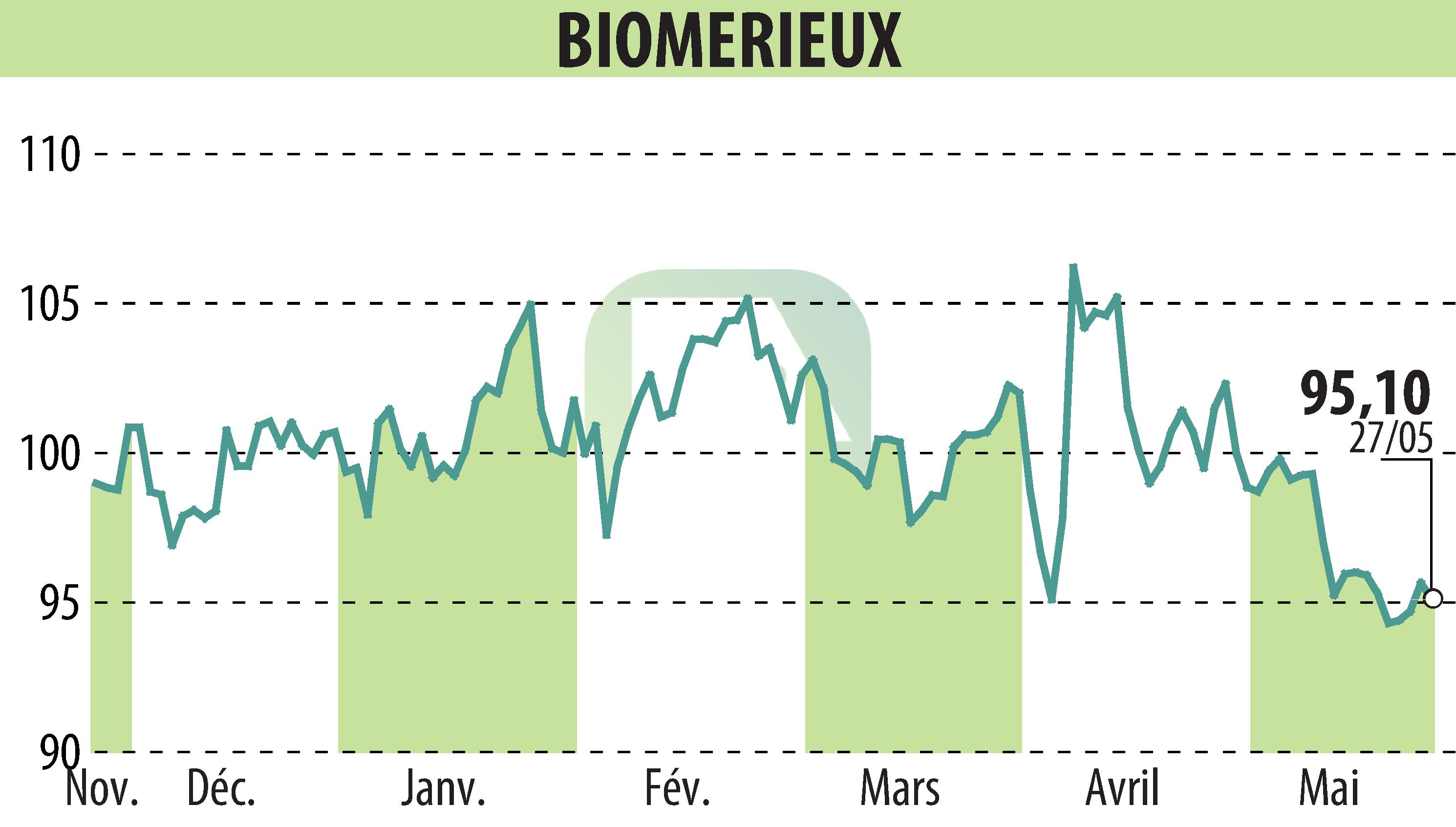
on BIOMERIEUX (EPA:BIM)
BioMérieux Receives FDA 510(k) Clearance for VIDAS® TBI Test
Marcy-l’Étoile (France), May 28th, 2024 – bioMérieux, a leader in in vitro diagnostics, has received U.S. FDA 510(k) clearance for VIDAS® TBI (GFAP, UCH-L1). This serum-based test supports the assessment of patients with mild traumatic brain injury (mTBI), including concussion. The test reduces the number of unnecessary head CT scans by predicting the absence of acute intracranial lesions.
Traumatic brain injury (TBI) is a major public health issue, affecting around 69 million people globally each year. For clinicians, determining the presence of life-threatening complications is crucial. Mild TBIs are the most prevalent, accounting for 70-90% of all TBI cases. However, 90% of mTBI patients who undergo CT scans have no abnormal findings. VIDAS® TBI (GFAP, UCH-L1) helps to avoid these unnecessary scans, saving time and resources.
Colin Hill, General Manager, North America, stated, “FDA clearance for VIDAS® TBI marks a significant milestone. It enables clinicians to perform fast and efficient triage for mTBI, leading to more informed treatment decisions.”
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOMERIEUX news
