on BIOMERIEUX (EPA:BIM)
BioMérieux Receives US FDA 510(k) Clearance for VITEK® REVEAL™ AST System
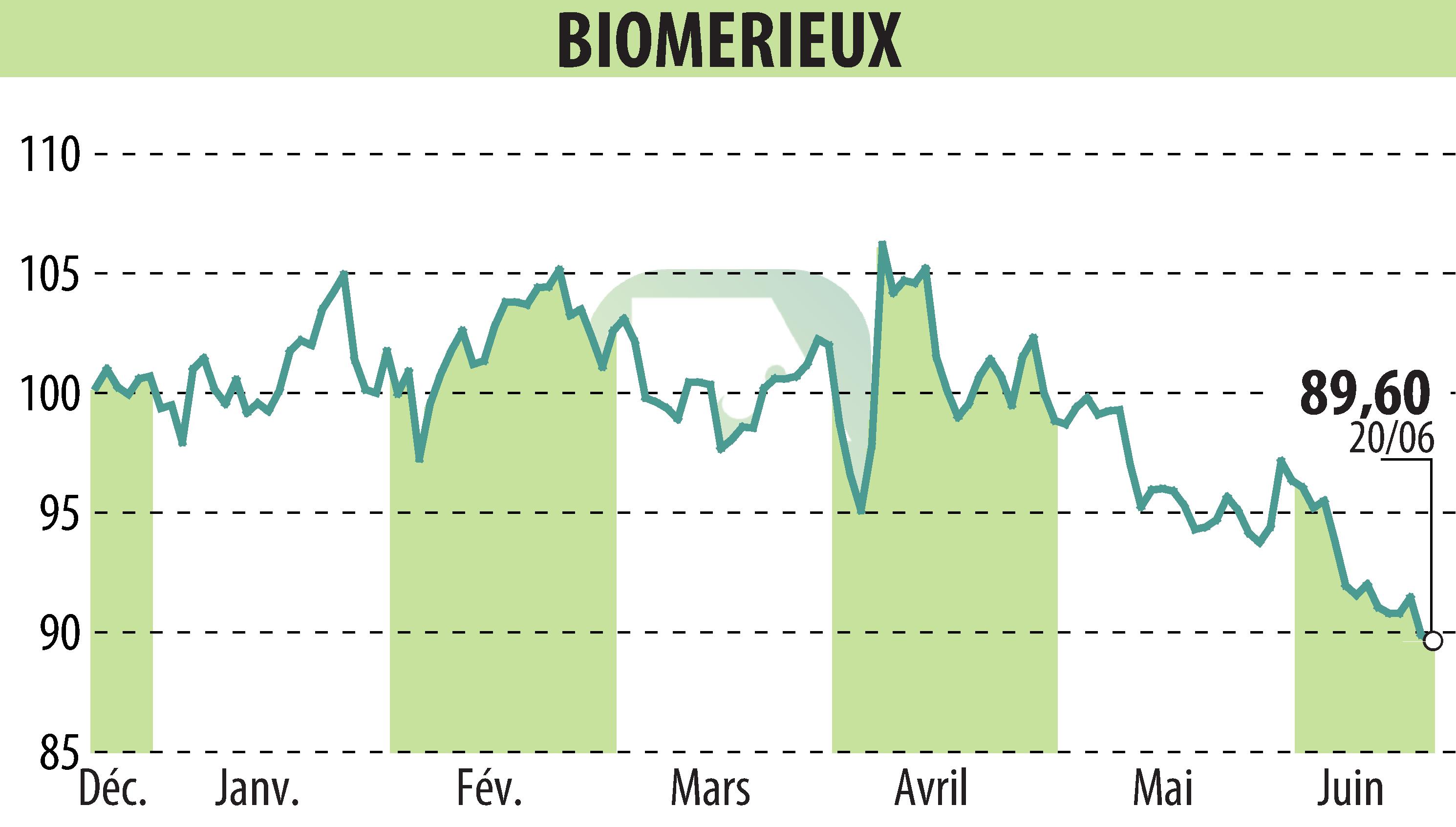
Marcy l’Étoile, France – June 21st, 2024 – bioMérieux has announced that its VITEK® REVEAL™ AST System has received U.S. FDA 510(k) clearance. This system provides antimicrobial susceptibility testing (AST) results directly from positive blood cultures, aiding clinicians in optimizing therapy for patients with bacteremic sepsis.
Worldwide, sepsis claims 11 million lives annually, with 1.3 million deaths linked to antibiotic-resistant bacteria. Rapid AST results are essential for improving patient care and supporting antimicrobial stewardship programs. These programs aim to reduce antimicrobial resistance, a global issue identified by the WHO.
The VITEK® REVEAL™ AST system, delivering results within 5.5-6 hours, is part of bioMérieux's strategy to combat sepsis and AMR. Acquired from Specific Diagnostics in 2022, this system integrates into bioMérieux’s diagnostic portfolio for bloodstream infections and sepsis.
This clearance allows the commercialization of VITEK® REVEAL™ in the U.S., following its Breakthrough Device Designation by the FDA in 2022. The system is also CE-marked in Europe under IVDD and IVDR regulations.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOMERIEUX news
