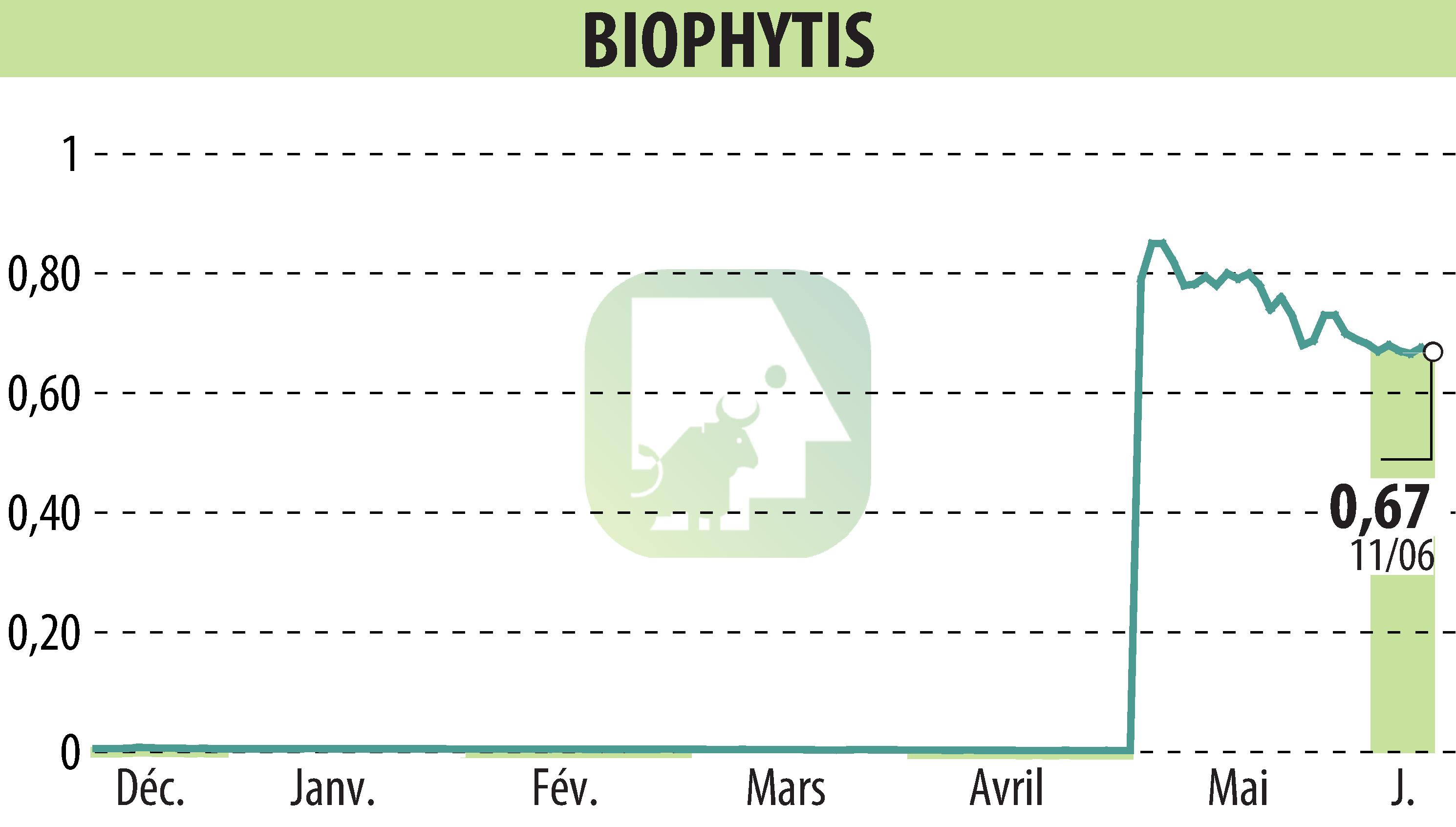
on Biophytis (EPA:ALBPS)
Biophytis announces the success of the industrial transfer of production of BIO101
Paris, France, Cambridge (Massachusetts, United States), June 12, 2024 – Biophytis, a biotechnology company specializing in the treatment of age-related diseases, announces the successful industrial transfer of BIO101 (20-hydroxyecdysone) carried out by its Seqens service provider.
Seqens, a global player in solutions for pharmaceutical markets, has produced the first batch compliant with Good Manufacturing Practices (GMP) of BIO101 in Villeneuve La Garenne. This batch is intended for Biophytis' clinical development program to treat respiratory deterioration in patients with Duchenne Muscular Dystrophy (DMD).
DMD is a rare genetic disease that causes muscle degeneration. BIO101 could improve the respiratory capacities and quality of life of non-ambulatory patients at an advanced stage of the disease.
Biophytis, after refining its protocol and obtaining orphan drug designation in Europe and the United States, is seeking partners to start a phase 1-2 clinical trial in DMD patients suffering from respiratory failure.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Biophytis news
