on Biophytis (EPA:ALBPS)
Biophytis Advances Obesity Treatment with EMA Approval
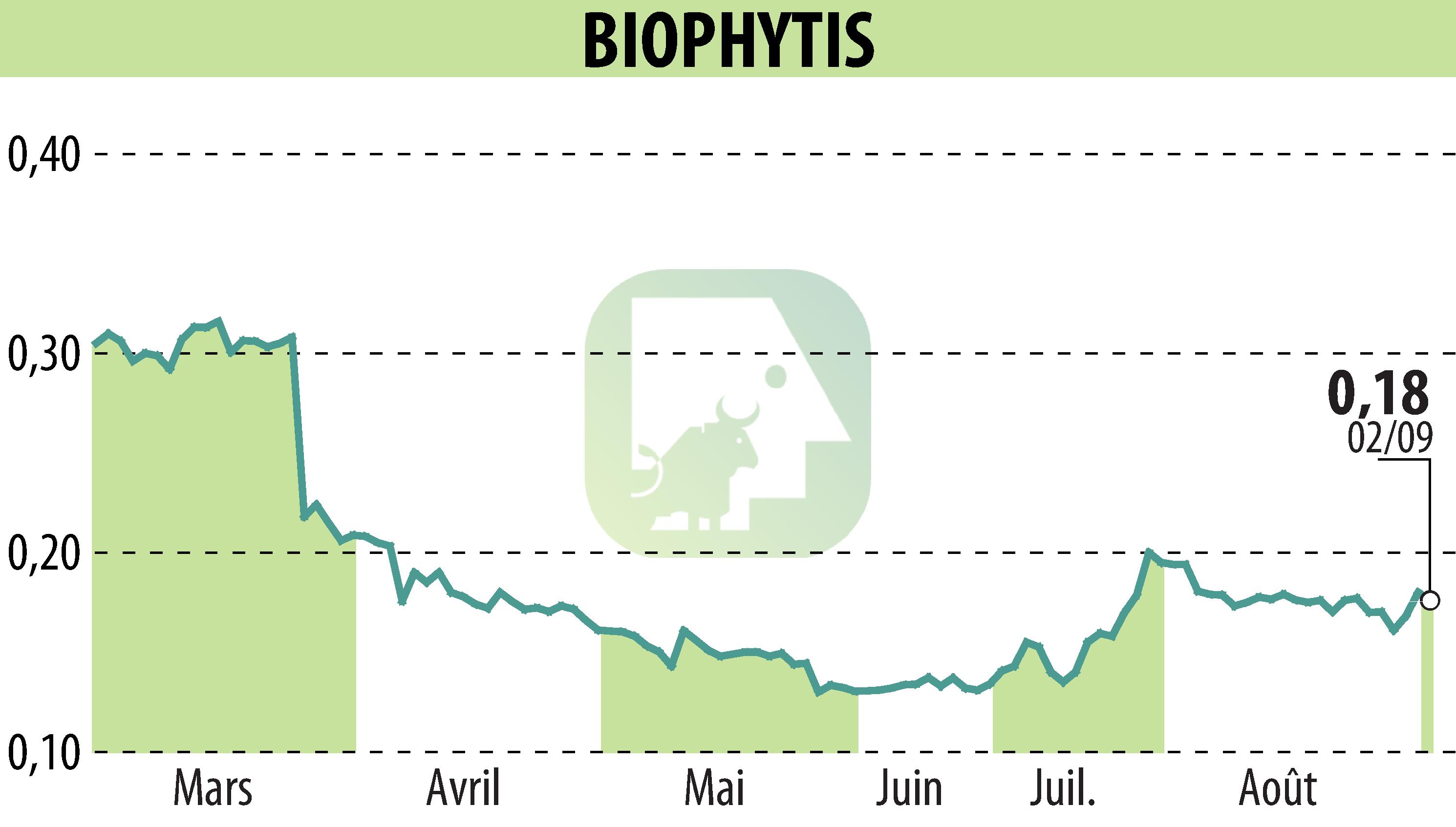
Biophytis SA has received authorization from the European Medicines Agency (EMA) to proceed with a Phase 2 clinical trial of BIO101, aimed at treating muscle wasting associated with obesity. This approval marks a vital step in Biophytis' plan to address this overlooked condition, which significantly contributes to long-term disability.
The Phase 2 trial will soon commence patient recruitment across Europe, following the completion of national ethical reviews. Biophytis CEO, Stanislas Veillet, emphasized the program's potential to meet critical medical needs in obesity-related muscle wasting.
In tandem with European efforts, Biophytis is preparing to extend its trial to Brazil, indicating an ongoing commitment to global regulatory strategies. The company continues to develop BIO101 for both muscular diseases and obesity.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Biophytis news
