on Biophytis (EPA:ALBPS)
Biophytis Files IND Application for Phase 2 Obesity Study with US FDA
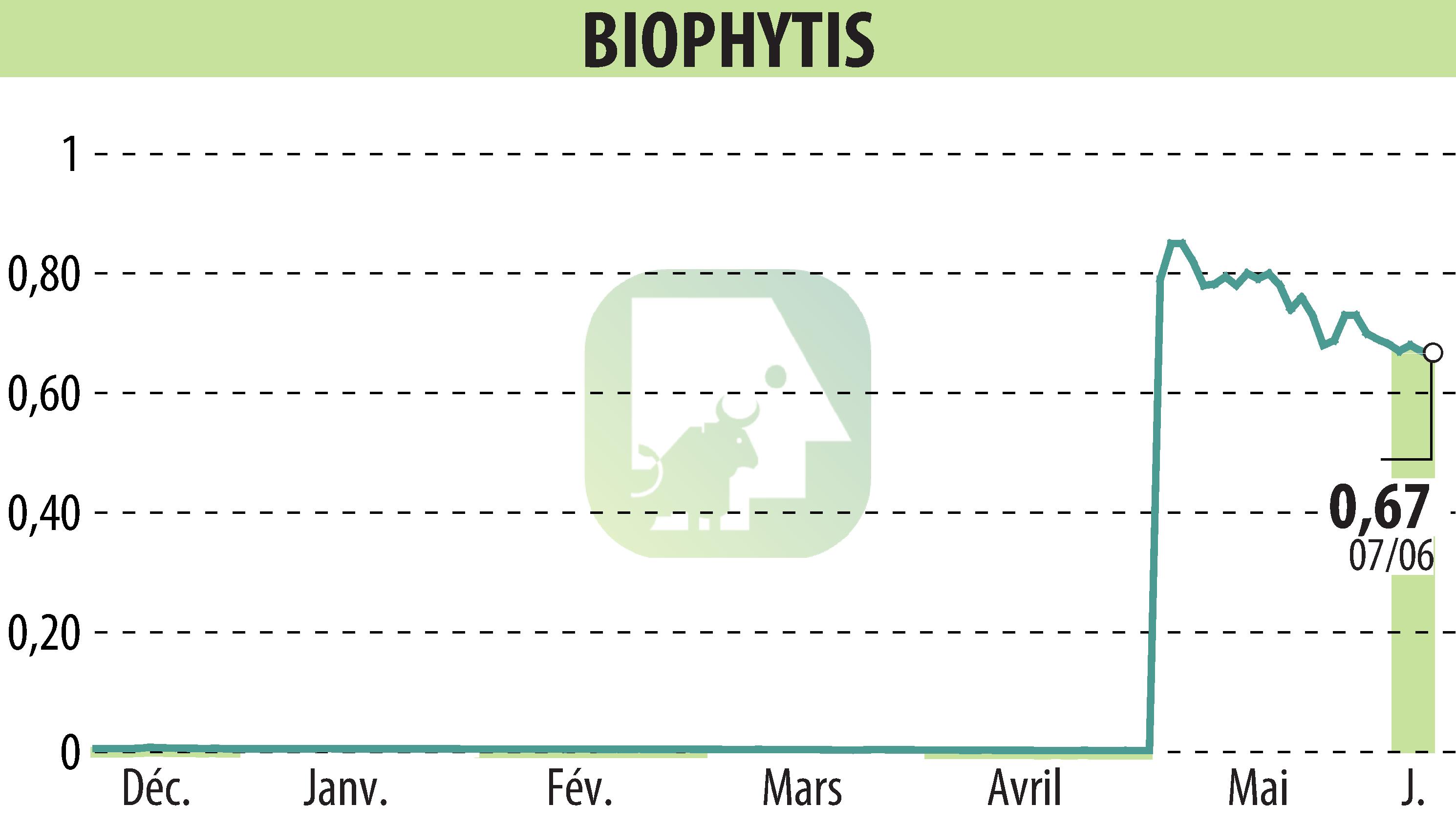
Paris (France) and Cambridge (Massachusetts, USA), June 10, 2024 - Biophytis SA, a clinical-stage biotechnology firm focusing on age-related disease therapies, has submitted an Investigational New Drug (IND) application to the US FDA for its phase 2 OBA clinical study targeting obesity.
The study will examine the efficacy and safety of BIO101 (20-hydroxyecdysone) in obese and overweight patients with secondary comorbidities, who will be treated for 21 weeks with GLP-1 receptor agonists and undergoing a hypocaloric diet. The primary objective is to evaluate improvements in muscle strength of the lower limbs. Secondary endpoints include mobility and body composition. The study is set to commence in mid-2024, pending FDA approval, with initial patient treatments expected later in the year.
Professor Marc-André Cornier, a recognized expert in obesity, will lead the study. Initial results are anticipated in 2025. Biophytis aims to expand research by including European clinical centers. CEO Stanislas Veillet emphasized the significant market potential for obesity treatments in the US.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Biophytis news
