on Biophytis (EPA:ALBPS)
Biophytis files IND application for phase 2 study in obesity
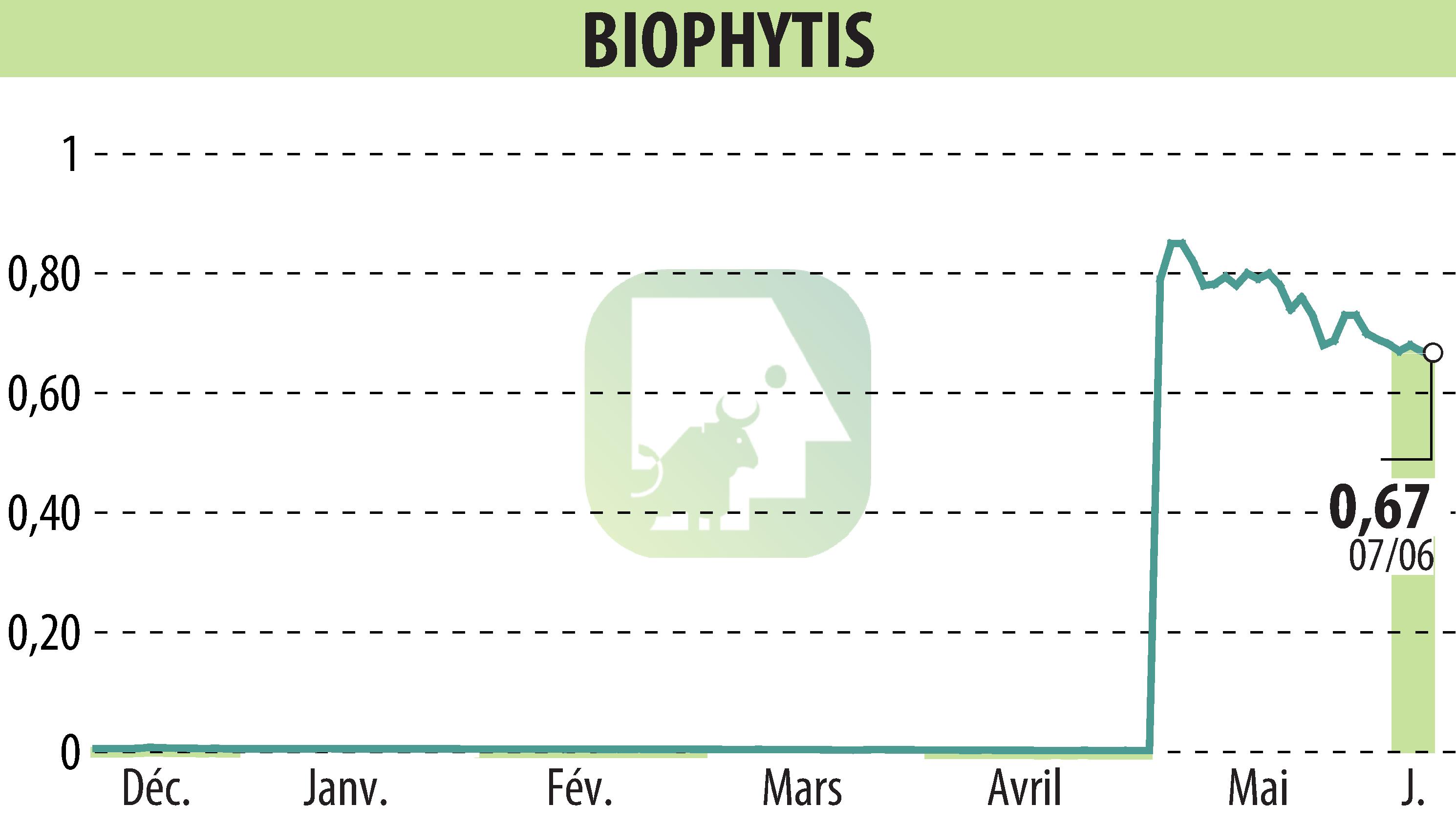
Paris, France and Cambridge, United States, June 10, 2024 – Biophytis SA, a biotechnology company, announces that it has filed an Investigational New Drug (IND) application with the FDA. This application is for a phase 2 study in obesity using BIO101 (20-hydroxyecdysone).
The OBA study will evaluate the effectiveness and safety of BIO101 in obese and overweight patients over a period of 21 weeks. Patients will also be treated with GLP-1 RAs and follow a low-calorie diet. The study will begin in mid-2024, subject to FDA approval.
Professor Marc-André Cornier, a recognized expert in the field of obesity, will be the principal investigator. Biophytis expects initial results in 2025. This initiative is expected to strengthen the company's position in the potential obesity market, estimated at $100 billion.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Biophytis news
