on BIOSYNEX (EPA:ALBIO)
BIOSYNEX obtains its first CE markings according to the new IVDR regulations
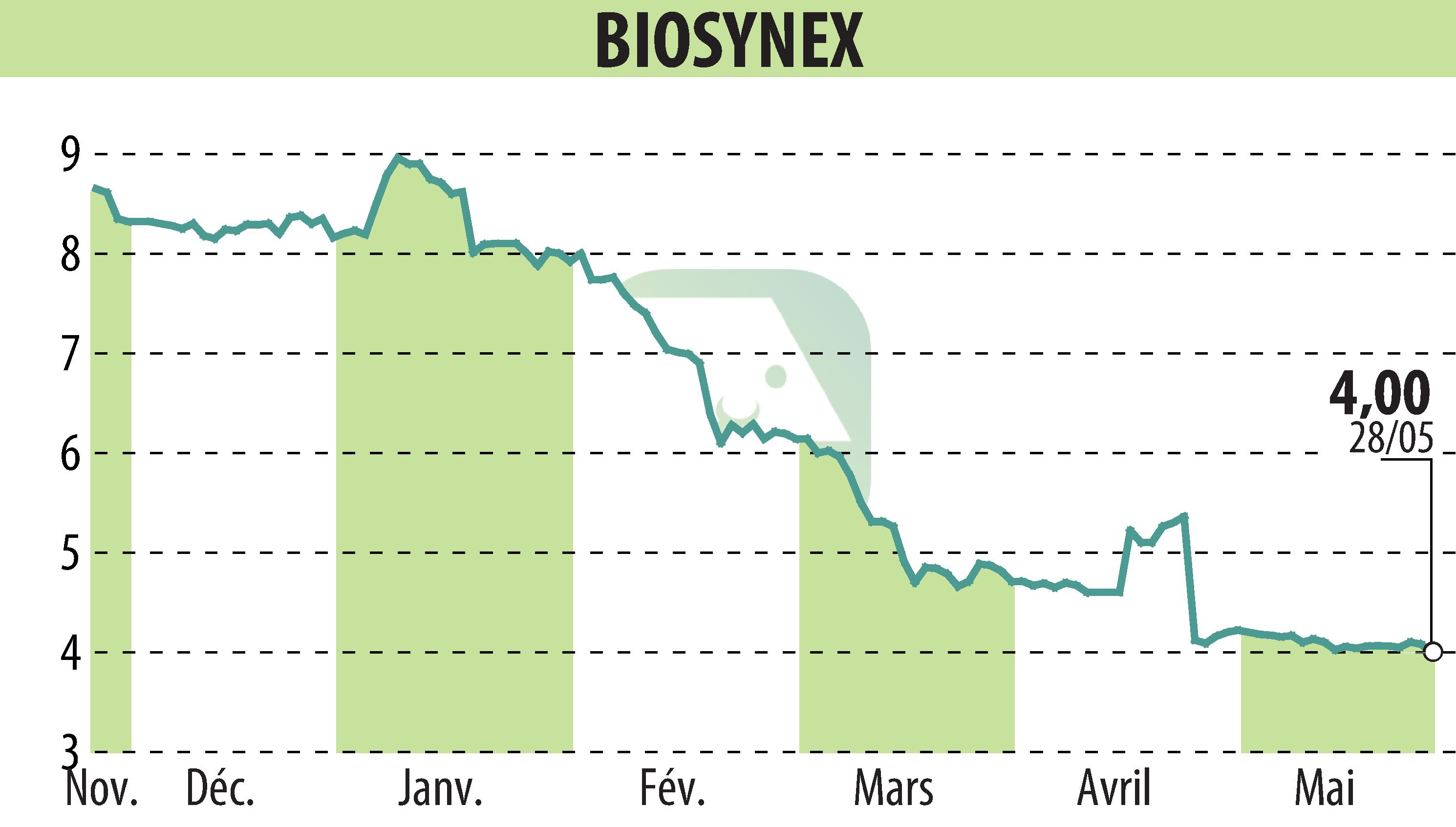
BIOSYNEX, a group specializing in rapid tests and molecular biology, announces that it has obtained its first CE markings according to IVDR regulations. This key step strengthens the compliance of its products with European legislation and confirms the company's know-how.
The markings concern Class B and C tests to detect infectious agents, some of which are sexually transmitted, as well as a coagulation test developed by AVALUN. This facilitates future certification of other similar products.
The American subsidiary CHEMBIO DIAGNOSTICS SYSTEMS also obtained CE IVDR marking for a rapid HIV detection test. This series of accreditations underlines BIOSYNEX's ability to operate within a strict regulatory framework, constituting an important competitive advantage.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOSYNEX news
