on Biotest AG (ETR:BIO)
Biotest's Yimmugo® Enters the U.S. Market
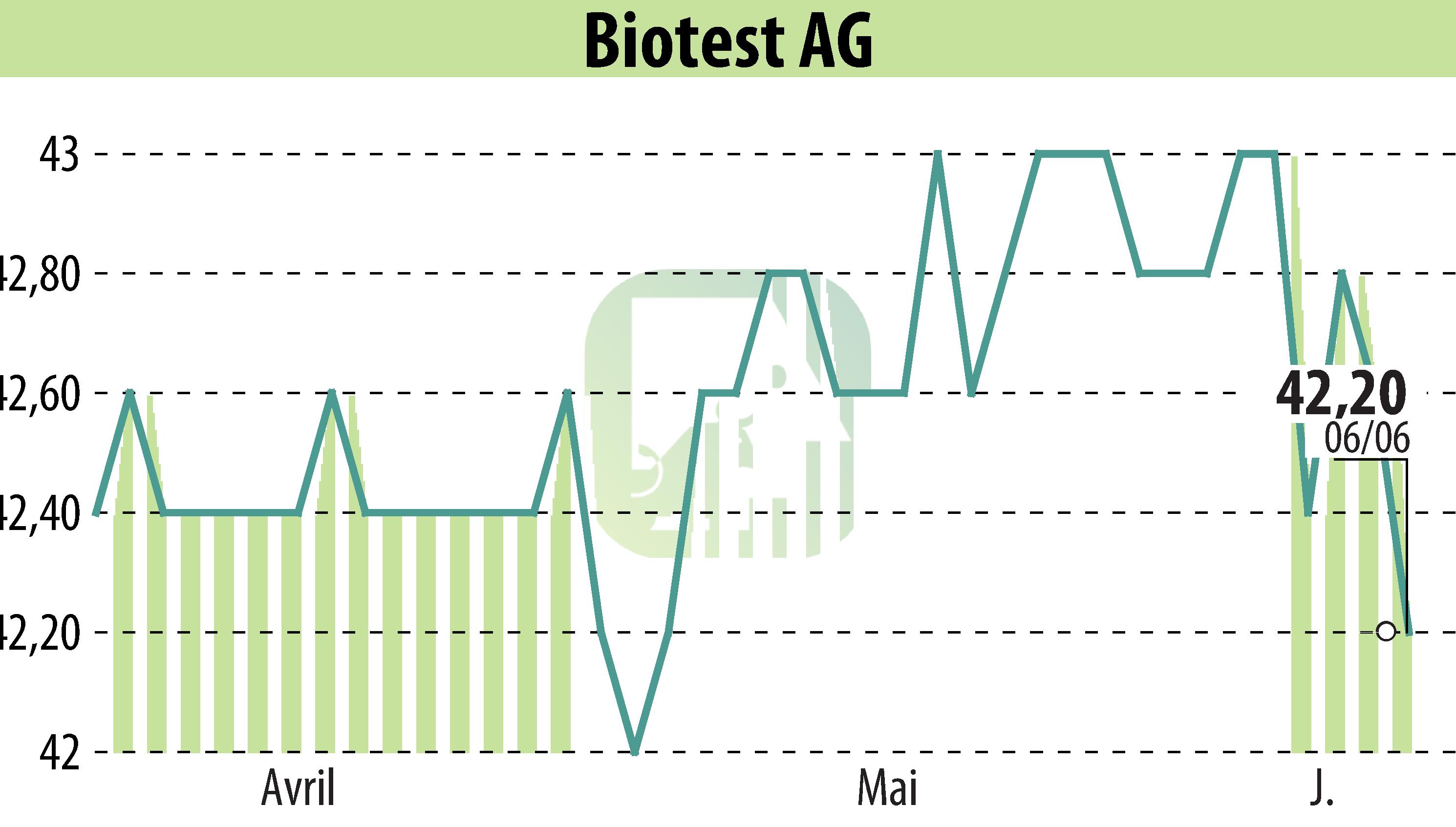
Biotest AG has announced the launch of Yimmugo®, an intravenous polyvalent human normal immunoglobulin, in the United States. This marks the first U.S.-approved medicine from Biotest, a company focusing on haematology, immunology, and intensive care medicine. The collaboration with Kedrion Inc. facilitates the distribution of Yimmugo® to patients with primary immunodeficiencies, affecting about 1 in 1,200 Americans.
Yimmugo® was initially introduced in Europe in 2022 and receives U.S. availability following FDA approval in 2024. Biotest’s state-of-the-art facility in Dreieich, Germany, underpins its production. The CEO of Biotest AG, Dr. Jörg Schüttrumpf, emphasizes the launch as enhancing U.S. patient access and extending their product reach.
This move aligns with Biotest's global expansion strategy, showcasing ongoing growth and innovation. It strengthens the company’s role in the plasma protein market, aiming to improve patient lives worldwide.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Biotest AG news
