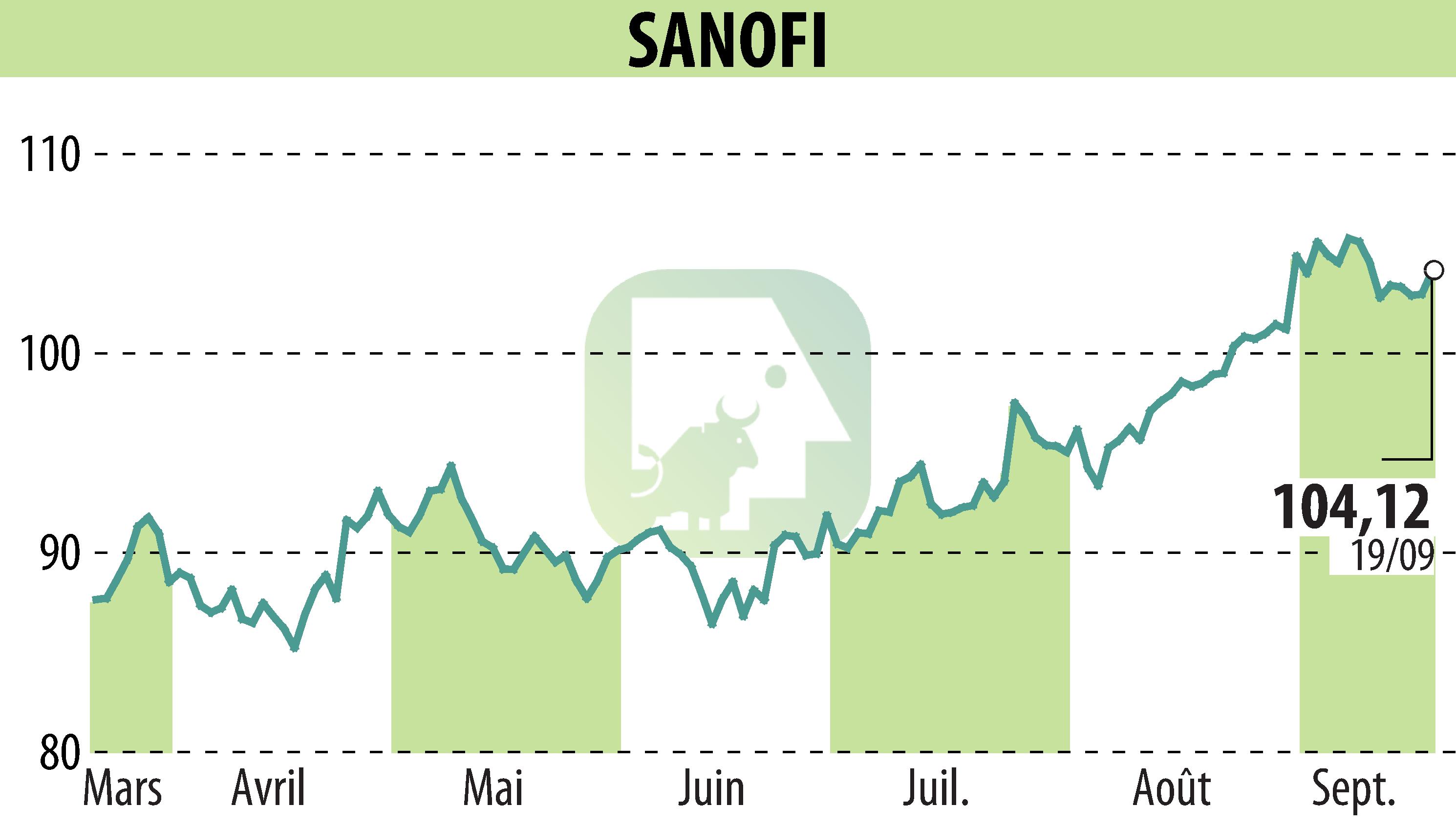
on SANOFI-AVENTIS (EPA:SAN)
CHMP recommends approval of Dupixent for children aged 12 months and older
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency has issued a positive opinion for the approval of Dupixent (dupilumab) in the European Union. This medicine is intended for the treatment of eosinophilic oesophagitis in children aged 12 months to 11 years.
This support is based on a phase III study, showing significantly greater histological remission in children treated with Dupixent compared to placebo. Dupixent is already approved for adults and adolescents over 12 years of age.
If approved, Dupixent would become the only medicine indicated in the EU to treat this condition in this age group. The results of the study show improvements in the symptoms of oesophagitis, with a safety profile consistent with that observed in adults and adolescents.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
