on CROSSJECT (EPA:ALCJ)
Crossject Achieves Key ZEPIZURE® Manufacturing Milestone
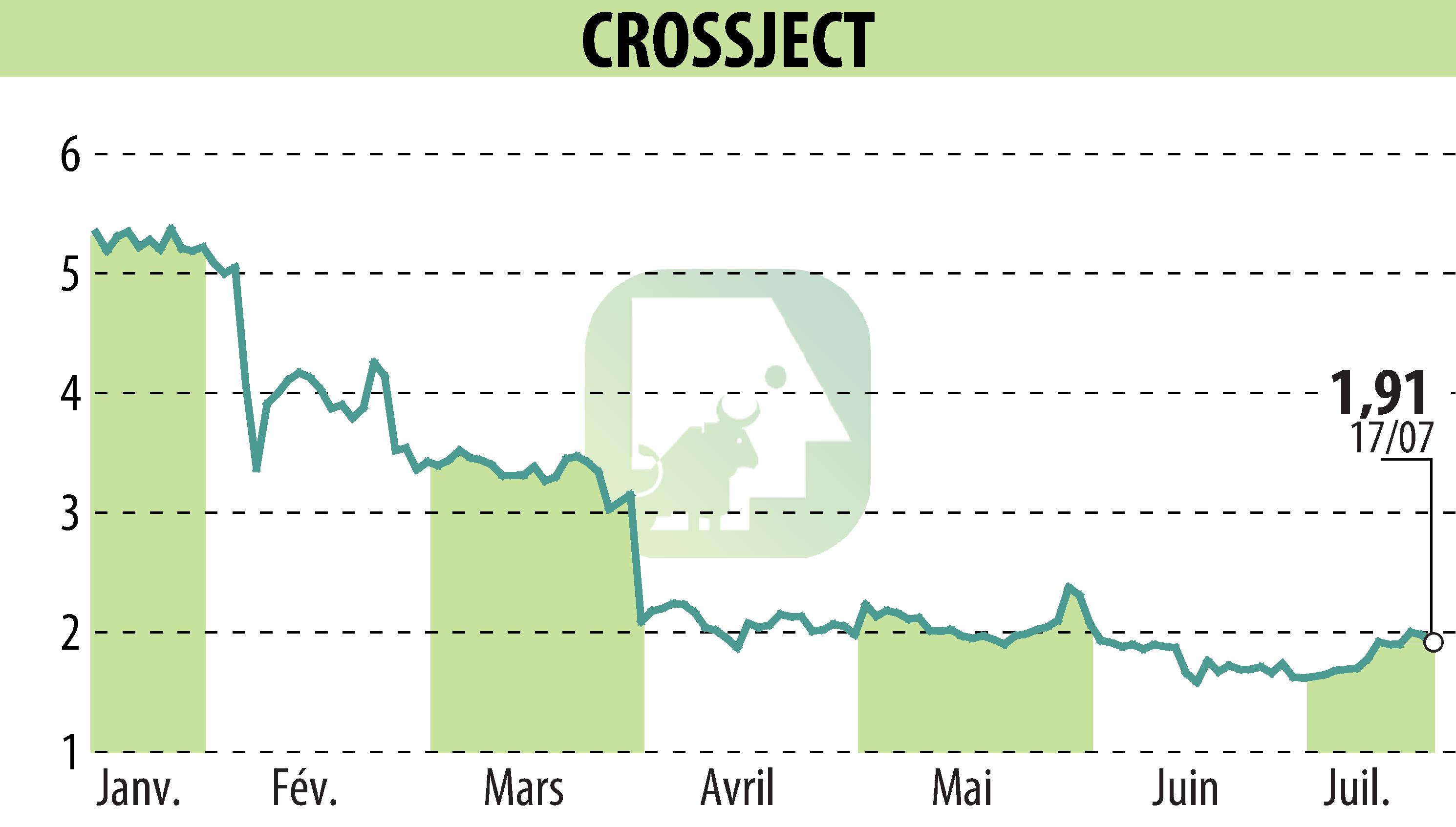
Crossject, a specialty pharma company, has announced the successful completion of a new registration batch of ZEPIZURE® at Eurofins' qualified facility. This site is designated as the Contract Development and Manufacturing Organization (CDMO) for the U.S. Biomedical Advanced Research and Development Authority (BARDA) deliveries.
This milestone builds upon successful results from a previous batch produced in December 2023. It is part of an ongoing manufacturing program aimed at confirming various parameters, including the shelf-life of ZEPIZURE®.
The completion of these manufacturing batches is crucial for the dossier that Crossject will submit to the U.S. Food and Drug Administration (FDA) to support the Emergency Use Authorization (EUA) application for ZEPIZURE®. Additionally, new data gathered from these batches will support future New Drug Application (NDA) filings from 2025 onward.
Patrick Alexandre, CEO of Crossject, stated that the progress of setting up a new CDMO partner and increasing fill-and-finish capabilities for ZENEO® is promising. These advancements not only strengthen their regulatory standing but also pave the way for the broad deployment of ZENEO® technology in managing epileptic crises and other emergency situations.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all CROSSJECT news
