on CureVac (NASDAQ:CVAC)
CureVac Announces Dosing of First Participant in Phase 2 Influenza Study
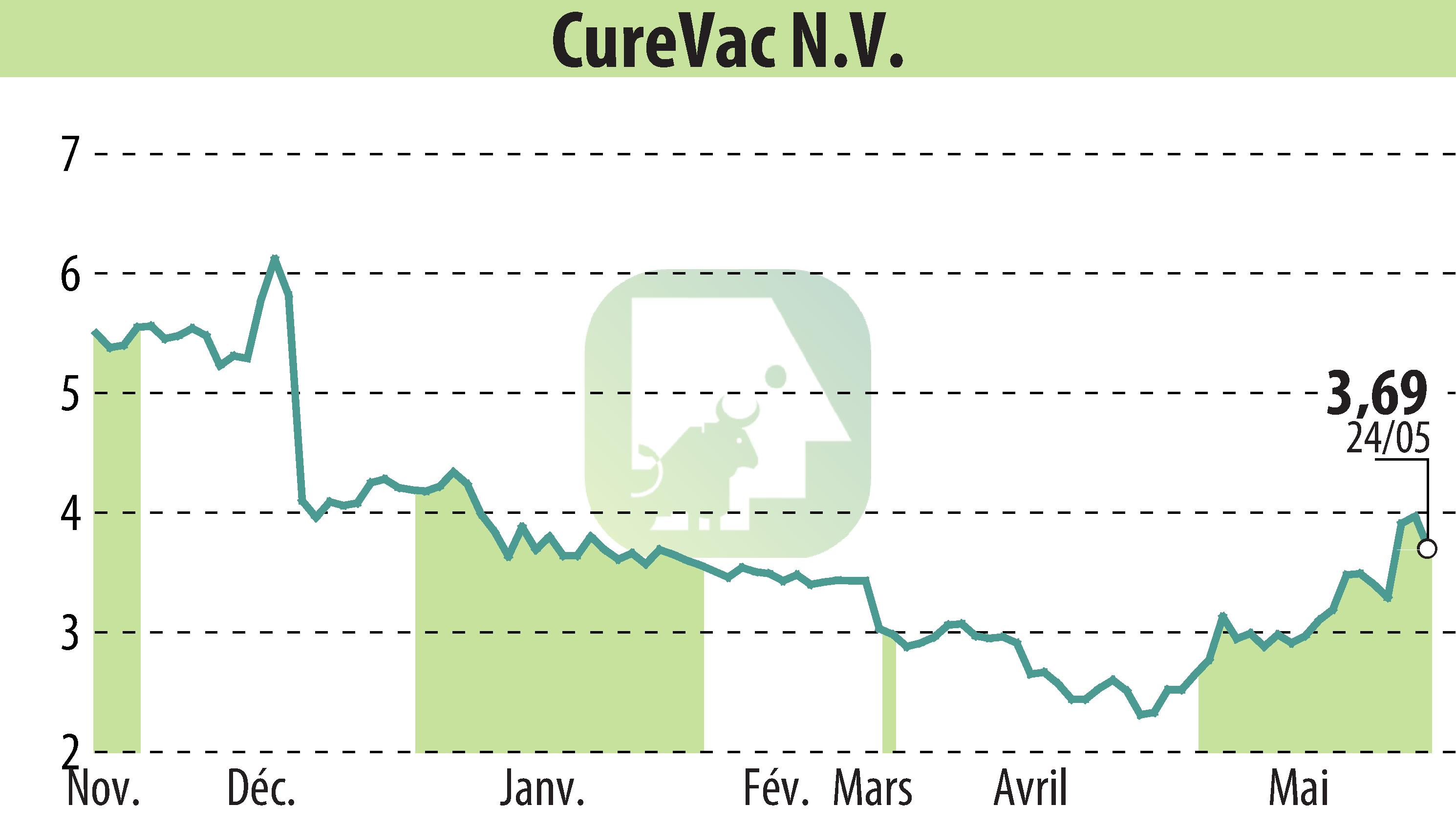
CureVac N.V. announced the dosing of the first participant in a Phase 2 study for their multivalent seasonal influenza vaccine candidate, developed in collaboration with GSK. The study aims to assess improved immune responses against influenza B strains.
Initiated after interim data from a Phase 1/2 study, the new Phase 2 trial follows the WHO's recommendation to exclude the B/Yamagata lineage, focusing now on two A strains and one B strain. The trial will test the vaccine candidate's safety, reactogenicity, and immunogenicity in 500 adults, aged 18 to 85, comparing it to licensed vaccines.
Dr. Myriam Mendila, Chief Scientific Officer of CureVac, stated that the interim data highlighted strong antibody responses, supporting the potential of CureVac's mRNA technology.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all CureVac news
