on DBV TECHNOLOGIES (EPA:DBV)
DBV's Viaskin Peanut Patch Secures Regulatory Progress in US and EU
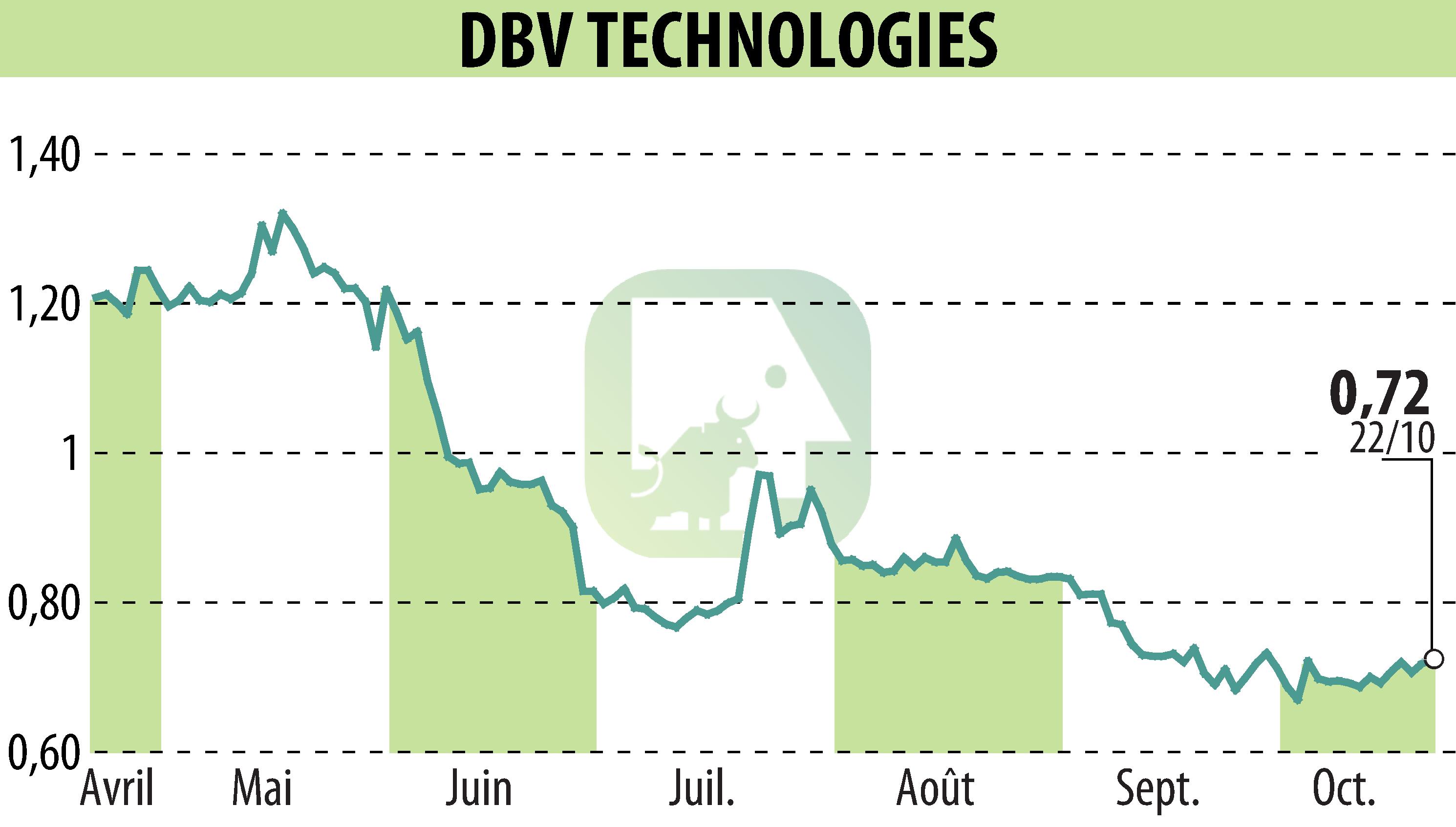
DBV Technologies has announced advancements for its Viaskin Peanut patch. The US FDA has agreed to an Accelerated Approval pathway for toddlers aged 1-3. A supplementary safety study for this group will start in Q2 2025. In Europe, the EMA confirmed the registration path for children aged 1-7. The company aims to pursue formal FDA approval through a BLA submission post-safety study.
The Phase 3 VITESSE study, evaluating children aged 4-7, exceeded initial enrollment targets with results anticipated by Q4 2025. The EMA's guidance supports a single submission for a 1-7 year-old indication. Despite positive updates, DBV reports financial challenges, with cash sufficient only until early 2025. Additional capital is being sought to continue development and potential launch efforts.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all DBV TECHNOLOGIES news
