on SANOFI-AVENTIS (EPA:SAN)
Dupixent Demonstrates Significant Efficacy in Bullous Pemphigoid
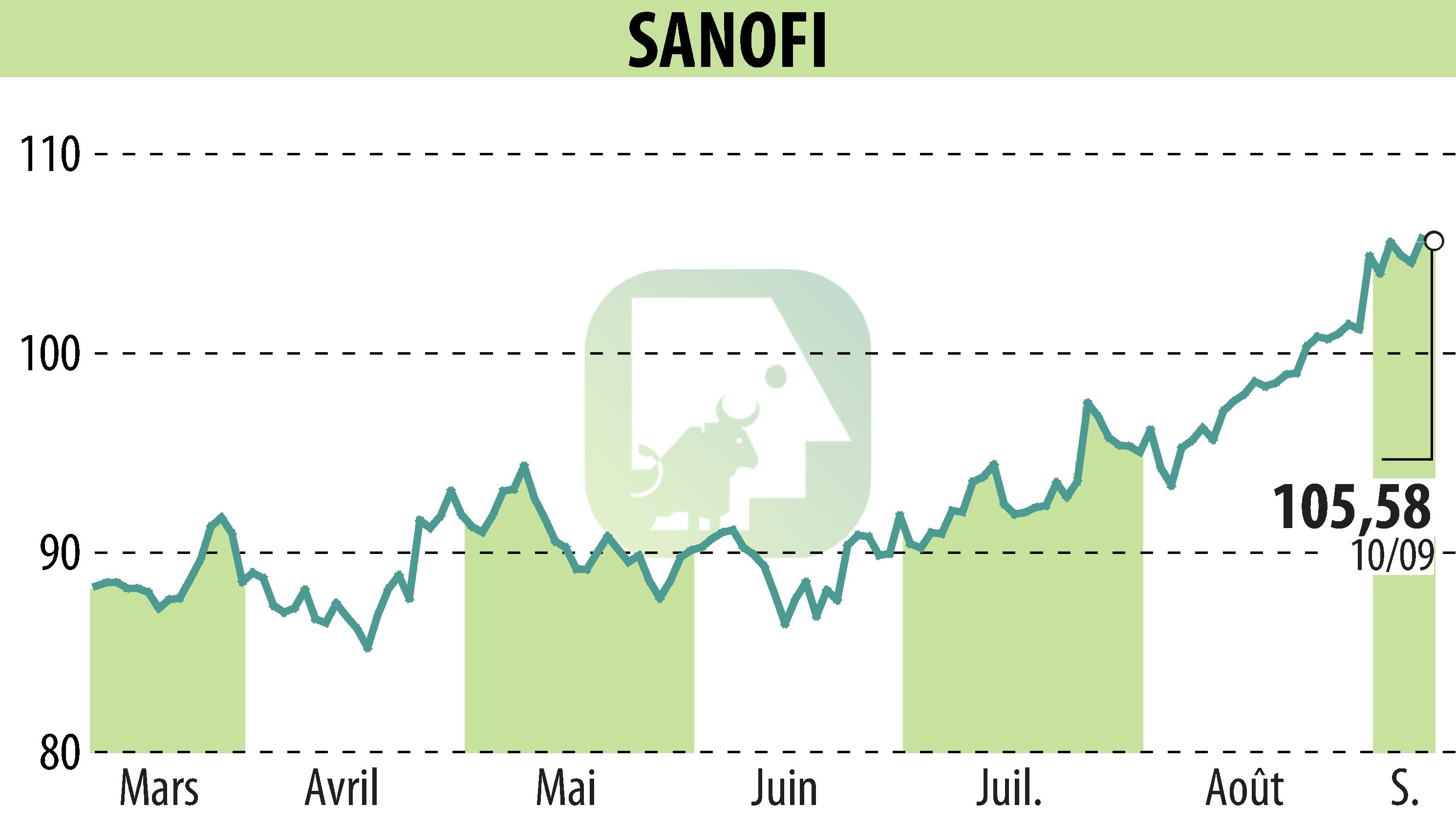
Sanofi and Regeneron announced that their biologic therapy, Dupixent (dupilumab), achieved significant improvements in disease remission and symptoms in patients with moderate-to-severe bullous pemphigoid (BP) during a pivotal study. The ADEPT study met its primary and all key secondary endpoints.
Dupixent showed a five-fold increase in sustained disease remission compared to placebo, achieving this primary endpoint in 20% of patients versus 4% for the placebo group. Additionally, Dupixent demonstrated a significant steroid-sparing effect. These results could make Dupixent the first targeted treatment for BP in both the U.S. and European Union.
BP is a chronic, relapsing disease characterized by intense itching, blisters, and painful lesions. This condition primarily affects elderly patients, often leading to serious complications.
The ADEPT study involved 106 adults, with patients receiving Dupixent or placebo every two weeks, alongside standard-of-care oral corticosteroids. Dupixent's efficacy was evident in various secondary endpoints, including a 77% reduction in disease severity and a 52% reduction in itch from baseline.
The safety profile of Dupixent aligned with previously observed data, with no deaths reported in the treated group. These promising results will support regulatory submissions globally, beginning in the U.S. later this year.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
