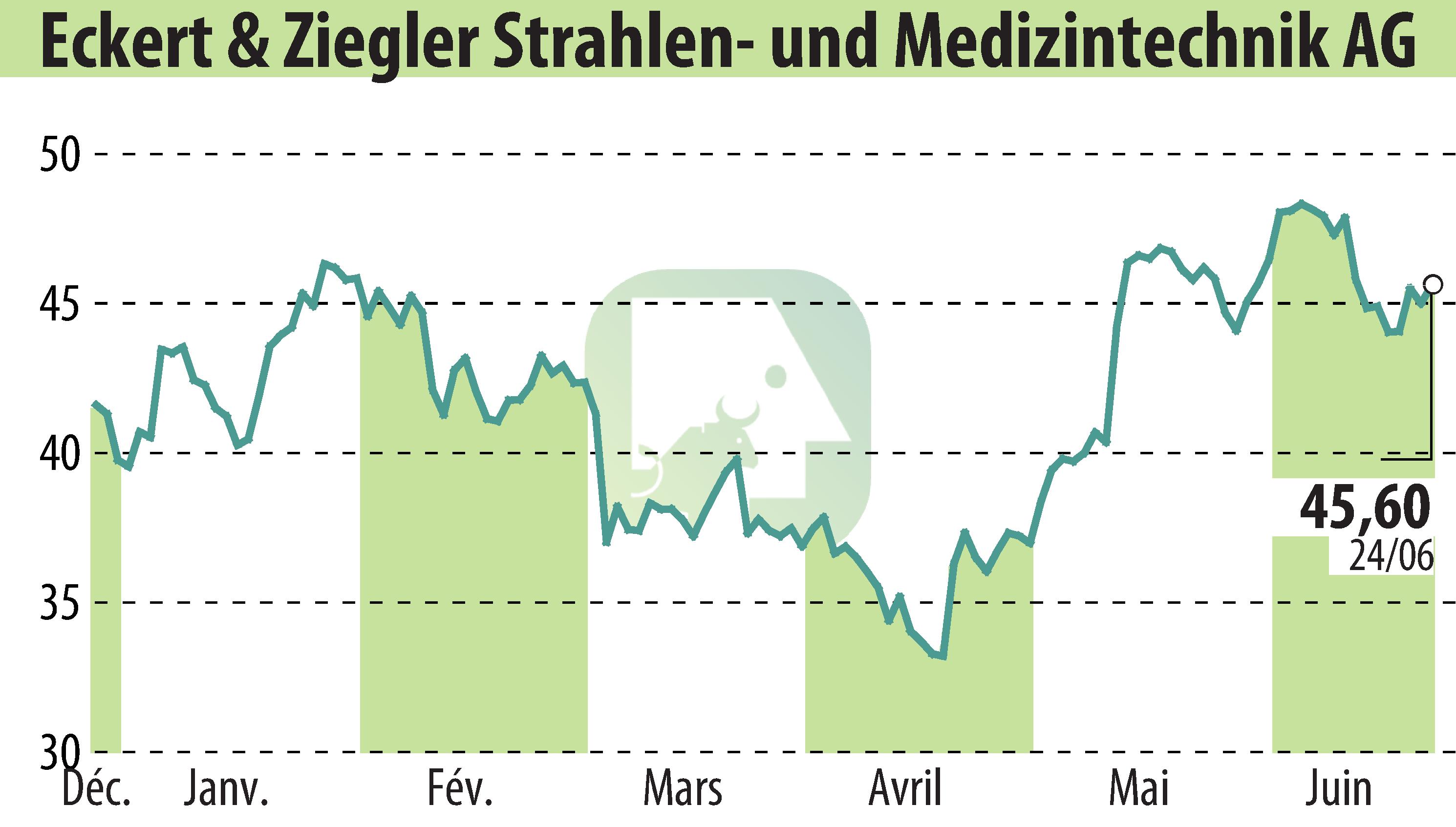
on Eckert & Ziegler Strahlen- Und Medizintechnik AG (isin : DE0005659700)
Eckert & Ziegler Subsidiary Pentixapharm Receives FDA Feedback to Initiate Phase III Trial with PentixaFor
Pentixapharm AG, a company owned by Eckert & Ziegler SE, has received positive feedback from the FDA to start a Phase III trial with the radiopharmaceutical Ga68-PentixaFor in Primary Aldosteronism (PA). PA, known as Conn’s syndrome, is a common cause of secondary hypertension.
The feedback from a recent Type C meeting suggests that existing clinical data may support the trial, potentially eliminating the need for a second clinical investigation. Ga68-PentixaFor meets criteria for fast track and breakthrough designation, accelerating its investigational new drug (IND) application.
Ga68-PentixaFor is a PET imaging tracer for detecting aldosterone-producing adenomas in PA patients. The approval could provide a non-invasive alternative to adrenal venous sampling, which is the current standard but is invasive and complex.
Dr. Dirk Pleimes of Pentixapharm stated that this milestone enables them to proceed with a US-centric Phase III trial. The aim is to improve diagnostic accuracy in Primary Aldosteronism and patient outcomes.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Eckert & Ziegler Strahlen- Und Medizintechnik AG news
