on Moderna, Inc. (NASDAQ:MRNA)
EMA Committee Recommends Marketing Authorization for Moderna's RSV Vaccine
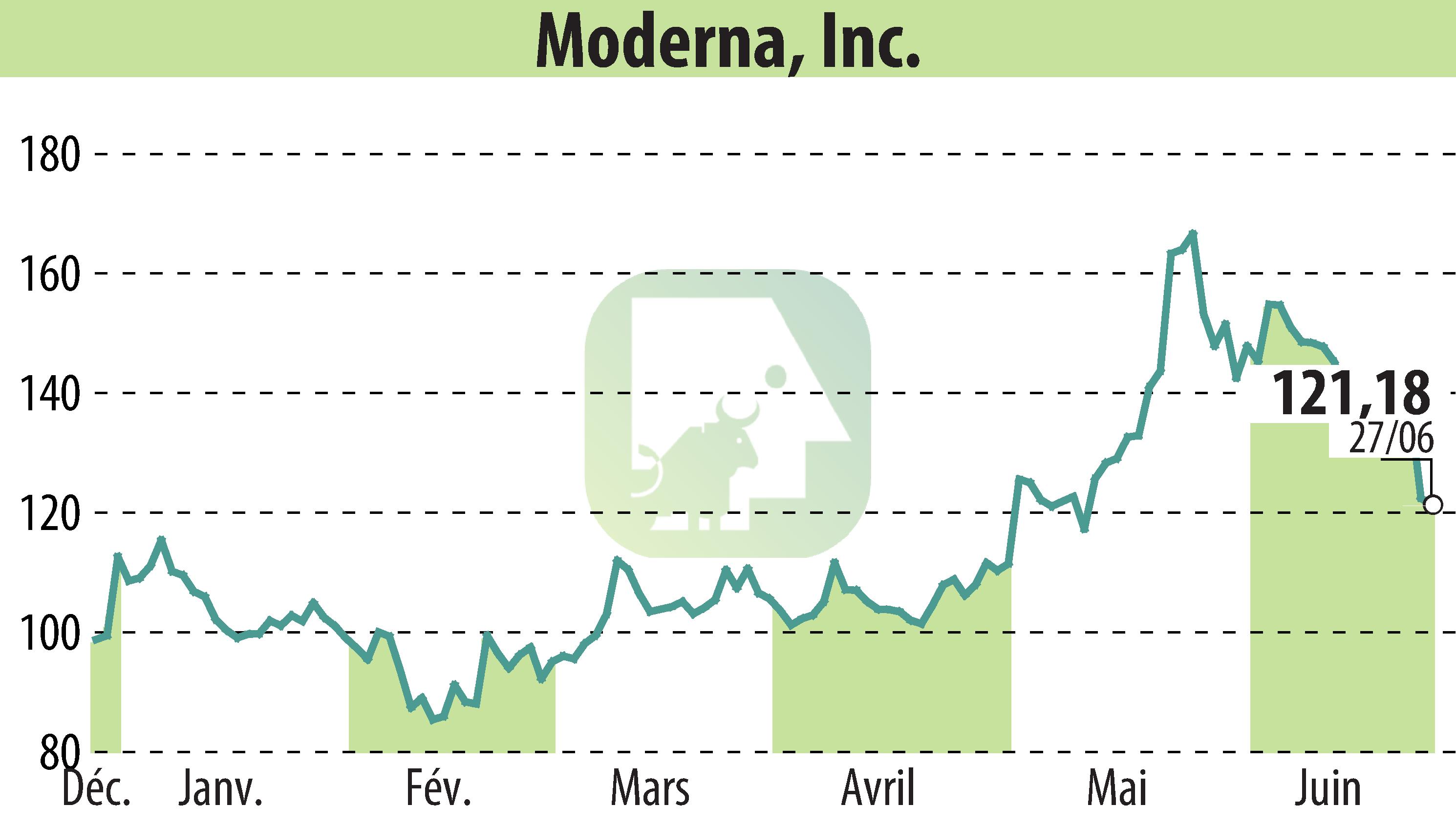
CAMBRIDGE, MA / ACCESSWIRE / June 28, 2024 / Moderna, Inc. (Nasdaq:MRNA) announced that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending the granting of marketing authorization in the European Union for mRESVIA (mRNA-1345), an mRNA respiratory syncytial virus (RSV) vaccine, to protect adults aged 60 years and older from lower respiratory tract disease caused by RSV infection. Following the CHMP's positive opinion, the European Commission will adopt a decision on the authorization of mRESVIA.
RSV is a highly contagious virus and a leading cause of lower respiratory tract infections and pneumonia. In the European Union, RSV causes about 160,000 hospital admissions in adults annually, with 92% of these admissions occurring in adults aged 65 and over.
The CHMP positive opinion is based on data from the Phase 3 clinical trial ConquerRSV, a global study conducted in approximately 37,000 adults aged 60 years or older in 22 countries. Results showed a vaccine efficacy against RSV lower respiratory tract disease of 83.7%, with durable efficacy maintained at 63.3% during follow-up. The most common adverse reactions were injection site pain, fatigue, headache, myalgia, and arthralgia.
In May 2024, the U.S. Food and Drug Administration (FDA) approved mRESVIA to protect adults aged 60 years and older from RSV infection. Moderna has filed for marketing authorization applications for mRESVIA in multiple markets worldwide.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
