on MEDESIS PHARMA (EPA:ALMDP)
Encouraging results from NanoLithium trial on Alzheimer's disease
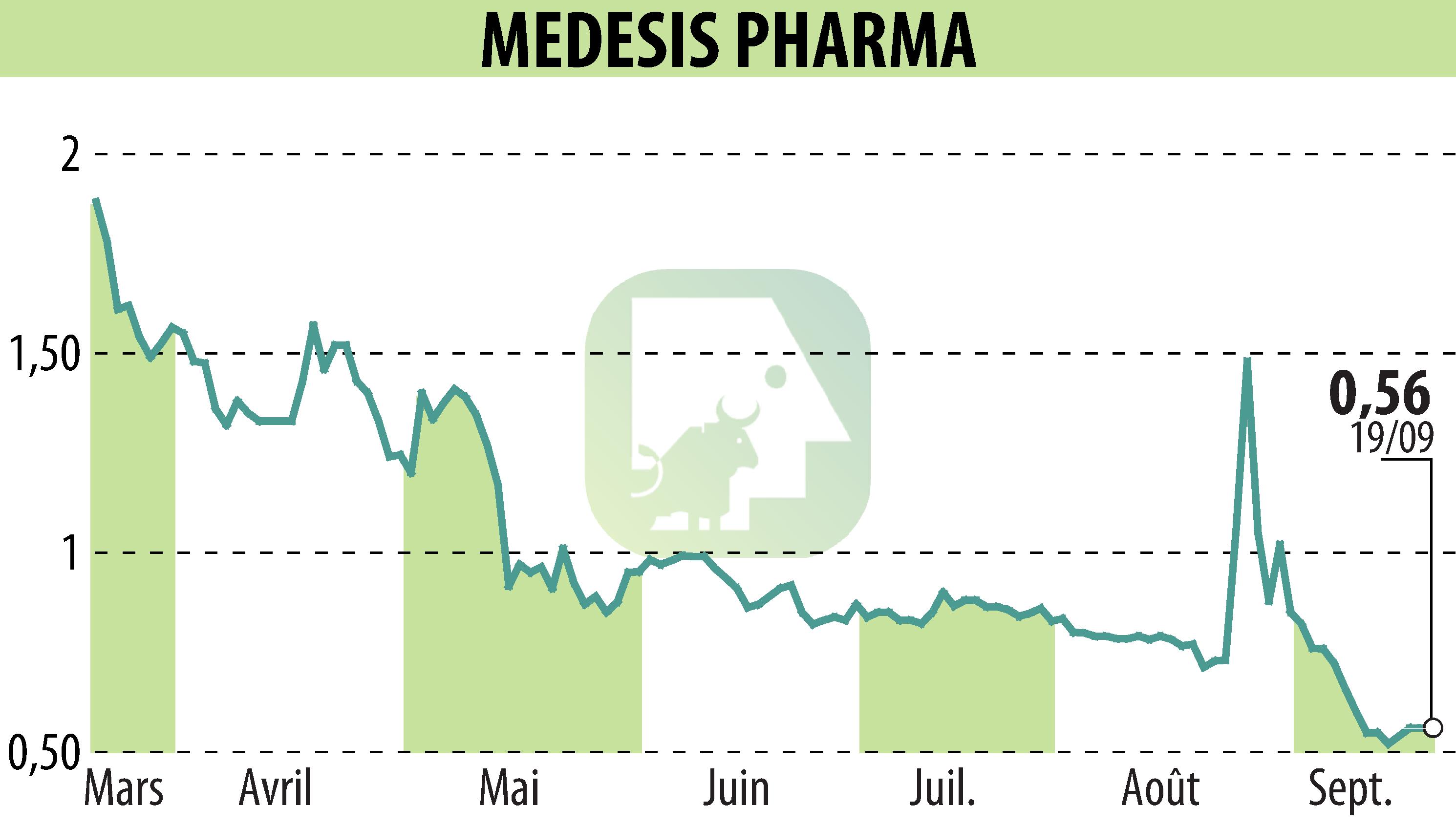
MEDESIS PHARMA announces positive results for the first phase of its phase 2a clinical trial on NanoLithium. The study, conducted on 68 patients with Alzheimer's disease, demonstrates good tolerance and significant clinical improvements compared to placebo.
In a commentary, Dr. Jean-Claude Maurel, CEO of Medesis Pharma, highlights the absence of notable side effects and a greater improvement in patients than that observed in the placebo group. Professor Jacques Touchon supports the continued development of NanoLithium to treat psycho-behavioral disorders associated with Alzheimer's disease.
The study was conducted in a double-blind fashion in eight centers in France, and the results will be followed by a second phase currently underway. Medesis Pharma is preparing to continue discussions with industrial partners for future regulatory clinical studies.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDESIS PHARMA news
