on EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Presents Positive One-Year Data at Annual Venous Forum
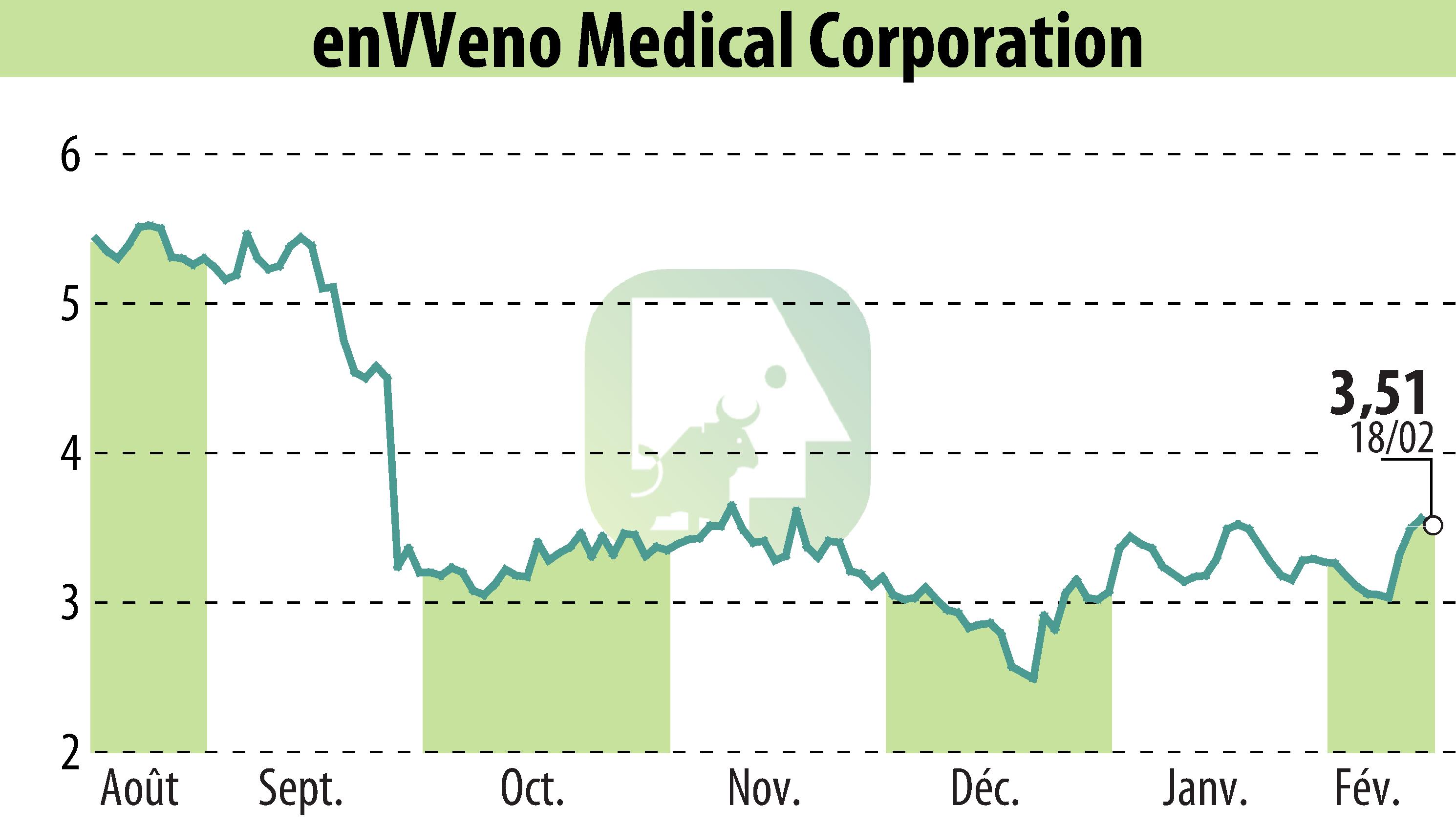
enVVeno Medical Corporation, an innovator in venous disease treatments, presented one-year follow-up data from the VenoValve U.S. pivotal trial. The results, showcased at the American Venous Forum's 37th Annual Meeting, highlight statistically significant improvements in patients' quality of life (QoL). The study involved 75 subjects who underwent the SAVVE procedure. The findings showed notable enhancements in venous disease-related QoL metrics over 12 months, confirmed by VEINS-SYM/QOL scores.
enVVeno has submitted a pre-market authorization (PMA) application for the VenoValve to the U.S. FDA, with a decision expected in late 2025. The VenoValve aims to be the first FDA-approved treatment for severe, deep venous chronic venous insufficiency (CVI), a condition with no current effective treatments. CVI, often caused by deep vein thromboses, affects daily life considerably, leading to high rates of depression and costing the U.S. healthcare system over $4 billion annually.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all EnVVeno Medical Corporation news
