on Nanohale AG (isin : DE000A1EWVY8)
FDA Approves Biosimilar FYB203/AHZANTIVE® for Retinal Diseases
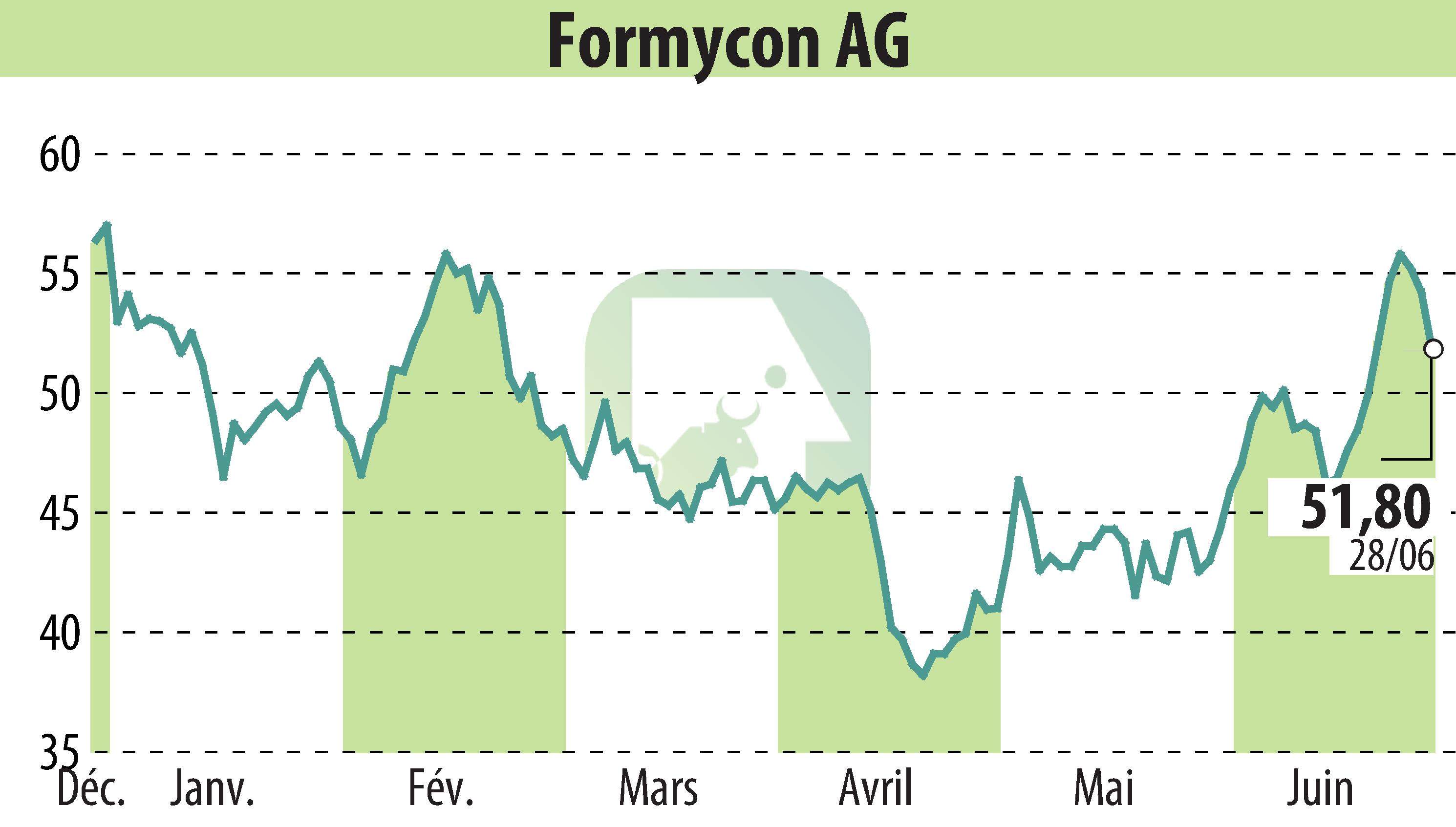
The U.S. Food and Drug Administration (FDA) has granted approval for FYB203/AHZANTIVE® (aflibercept-mrbb), a biosimilar to Eylea®. This announcement was made by Formycon AG and its licensing partner Klinge Biopharma GmbH.
FYB203/AHZANTIVE® is approved for treating neovascular Age-Related Macular Degeneration (nAMD), Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Macular Edema following Retinal Vein Occlusion (RVO). The decision follows a comprehensive evaluation of data demonstrating the biosimilar's comparable efficacy, safety, pharmacokinetics, and immunogenicity to Eylea®.
The thorough evaluation included analytical, pre-clinical, clinical, and manufacturing data, ensuring that FYB203/AHZANTIVE® meets the necessary standards for patient treatment in these serious retinal conditions.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
