on SANOFI-AVENTIS (EPA:SAN)
FDA Approves Qfitlia for Hemophilia A and B Treatment
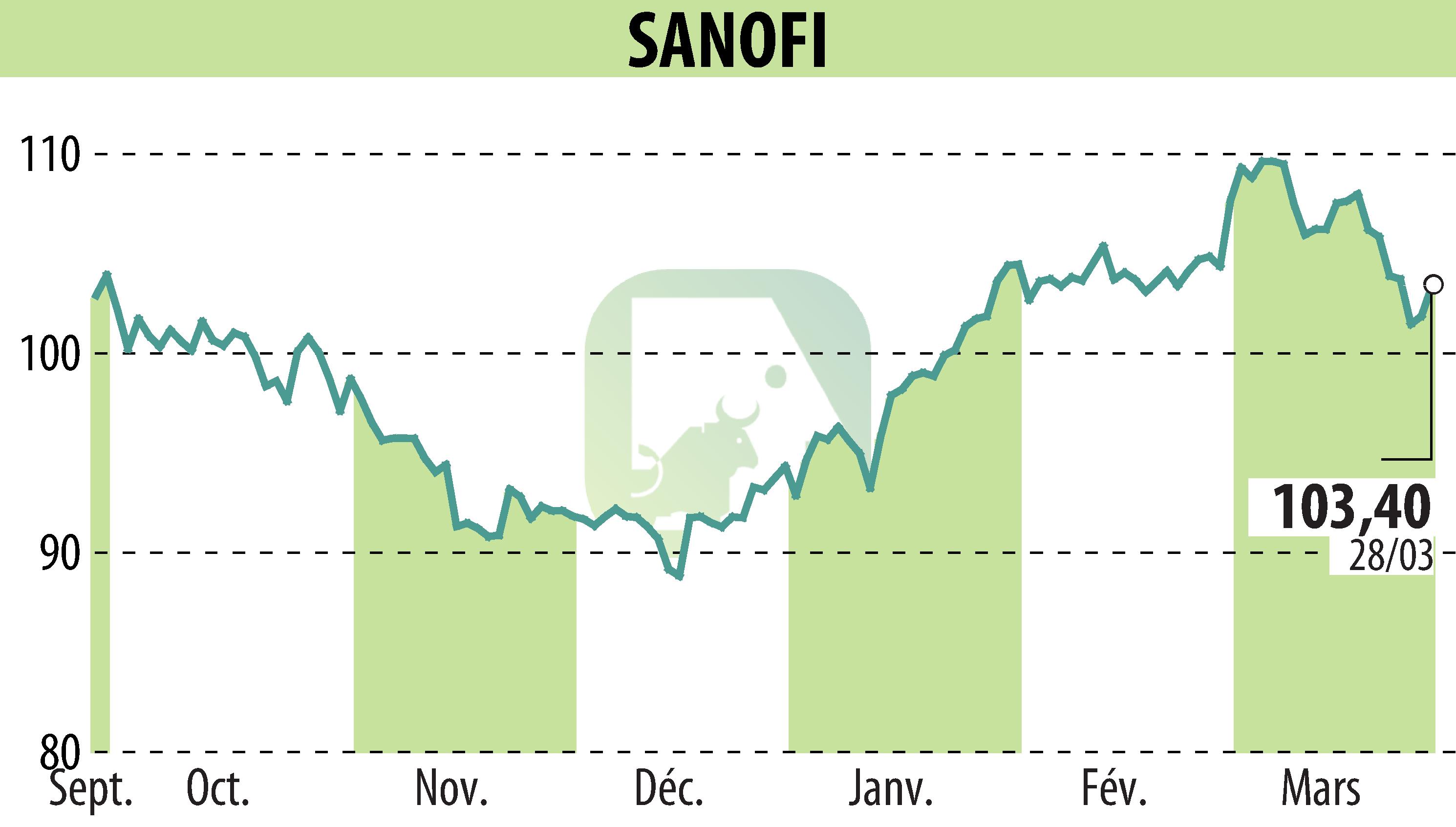
On March 28, 2025, the FDA approved Qfitlia (fitusiran), marking a significant milestone for hemophilia treatment in the US. Qfitlia is the first antithrombin-lowering therapy designed for hemophilia A or B patients, with or without inhibitors. The approval follows the ATLAS phase 3 studies, highlighting notable reductions in annualized bleeding rates (ABR) among different patient groups. Qfitlia's unique mechanism involves lowering antithrombin to enhance thrombin generation, thereby improving blood clotting.
Qfitlia requires as few as six injections annually, providing a less burdensome treatment schedule. Clinical trials revealed a significant 71% ABR reduction for patients without inhibitors compared to traditional therapies. However, potential adverse reactions include thrombotic events and infections.
The FDA has also approved a companion diagnostic assay to measure AT levels, enhancing treatment accuracy. Qfitlia is set to offer a comparable price to existing treatments, available with patient support services under the HemAssist program. Regulatory decisions in Brazil and China are anticipated in 2025.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
