on Nanohale AG (isin : DE000A1EWVY8)
Formycon Begins Phase III Clinical Trial for Keytruda® Biosimilar FYB206
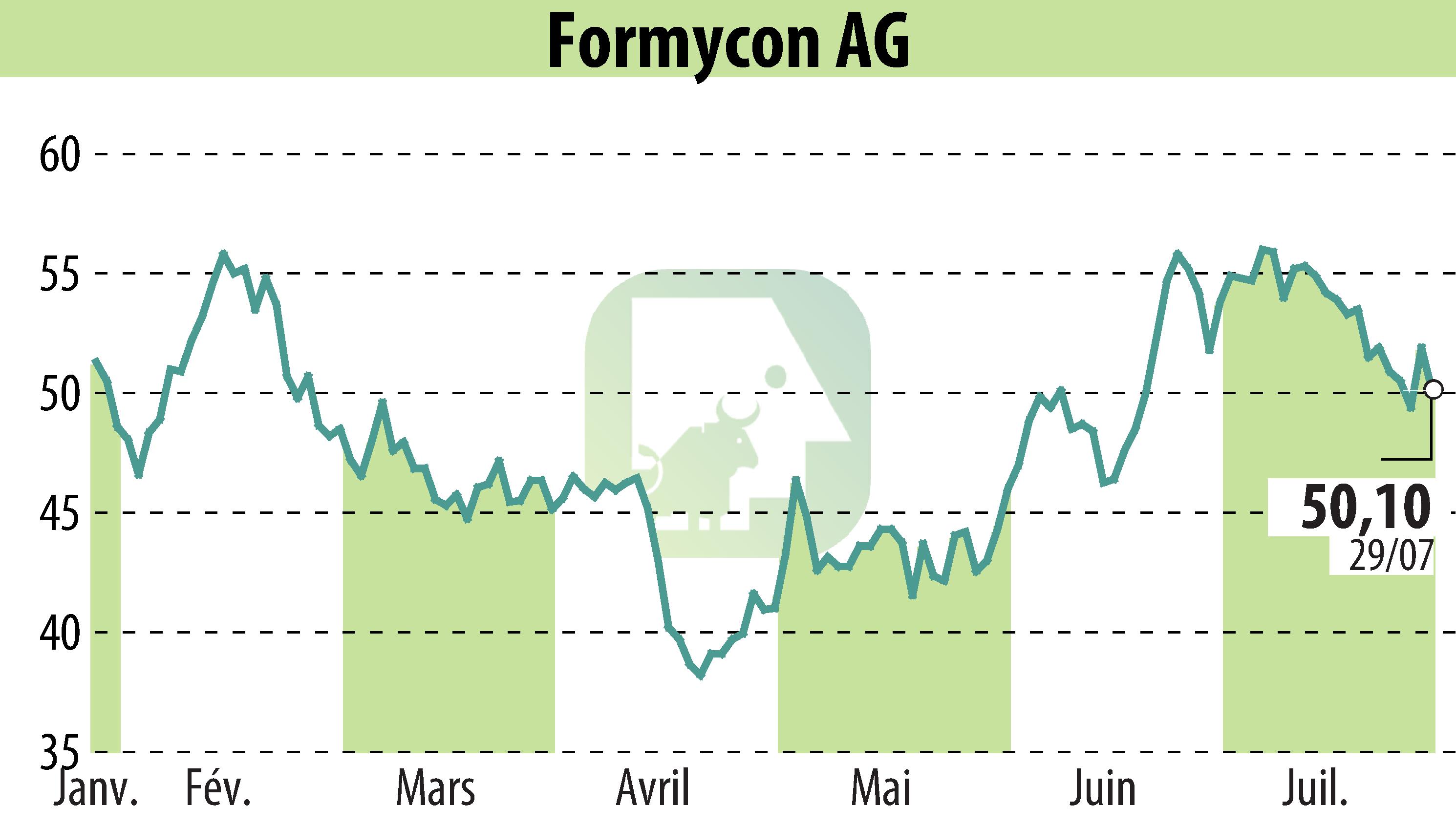
Formycon AG has announced the initiation of a Phase III clinical trial for its Keytruda® biosimilar candidate, FYB206. The trial, named "Lotus," will assess the safety and efficacy of FYB206 compared to Keytruda® (pembrolizumab) in approximately 500 patients with non-small cell lung cancer (NSCLC). The study spans several countries in Eastern Europe and Southeast Asia.
This double-blind, multicenter study will include up to 17 treatment cycles over 40 weeks, with subsequent therapy for an additional 12 months. The trial design was developed in coordination with the FDA, EMA, and PMDA. Preliminary results are anticipated in 2027.
FYB206 aims to provide a cost-effective treatment option post the market exclusivity expiration of Keytruda® in the USA by 2029 and the EU by 2030. This trial marks a significant milestone for Formycon in immuno-oncology.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
