on Nanohale AG (isin : DE000A1EWVY8)
Formycon Receives FDA Approval for Aflibercept Biosimilar
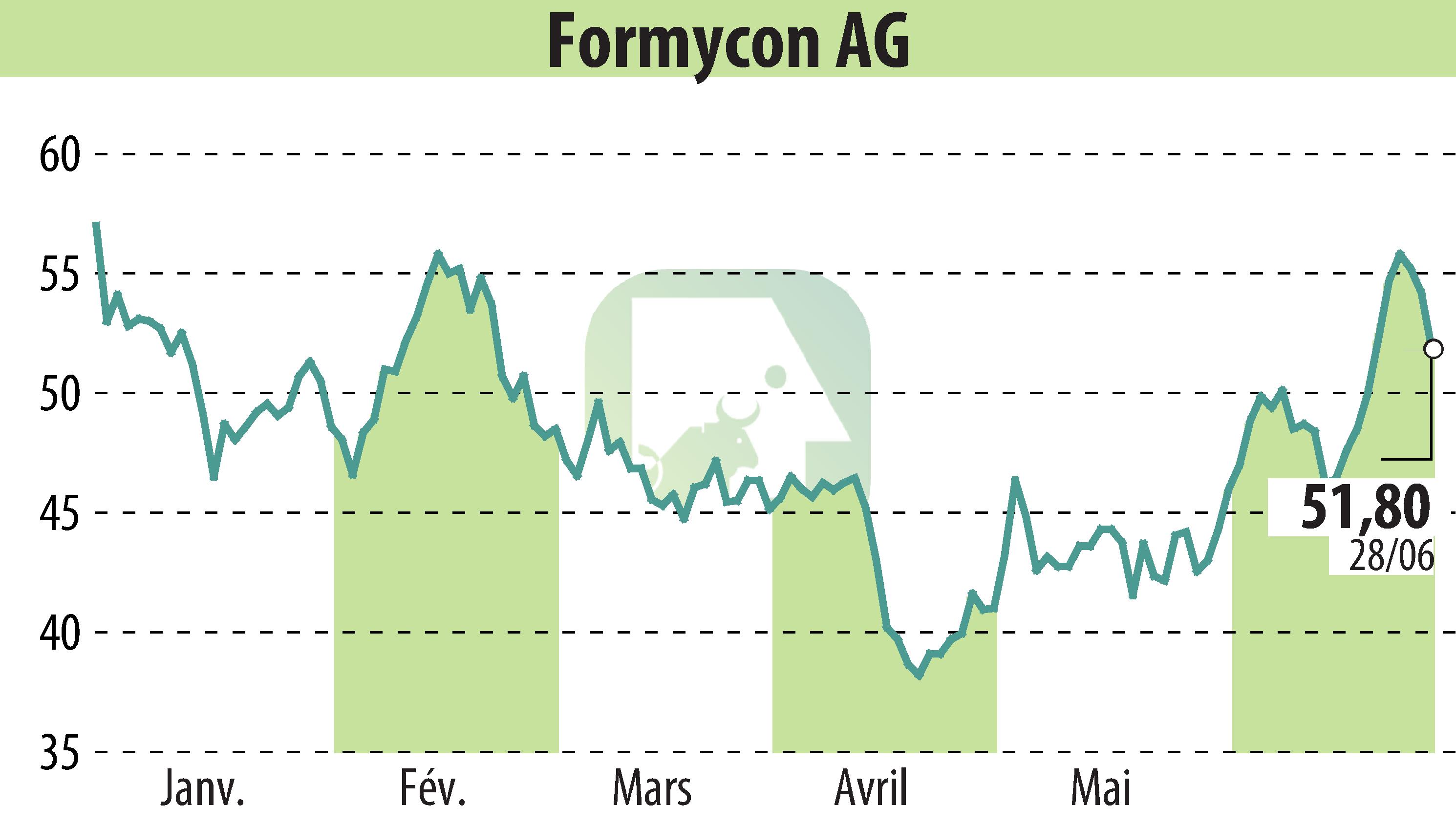
Formycon AG has announced that the U.S. Food and Drug Administration (FDA) approved its biosimilar, FYB203/AHZANTIVE® (aflibercept-mrbb), on June 28, 2024. This biosimilar is an alternative to Eylea®, used in treating various serious retinal diseases.
The approval marks a significant milestone for Formycon and its partner, Klinge Biopharma GmbH, confirming their position in ophthalmic biosimilar therapies. FYB203/AHZANTIVE® has demonstrated comparable efficacy and safety to Eylea® in clinical studies.
This biosimilar targets Eye-Related Neovascular (wet) Macular Degeneration (nAMD) and other retinal diseases like Diabetic Macular Edema and Diabetic Retinopathy. In 2023, Eylea® achieved global sales of approximately $9 billion, underlining the market potential for FYB203/AHZANTIVE®.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
