on Nanohale AG (isin : DE000A1EWVY8)
Formycon's FYB203 Biosimilar Receives Positive CHMP Opinion
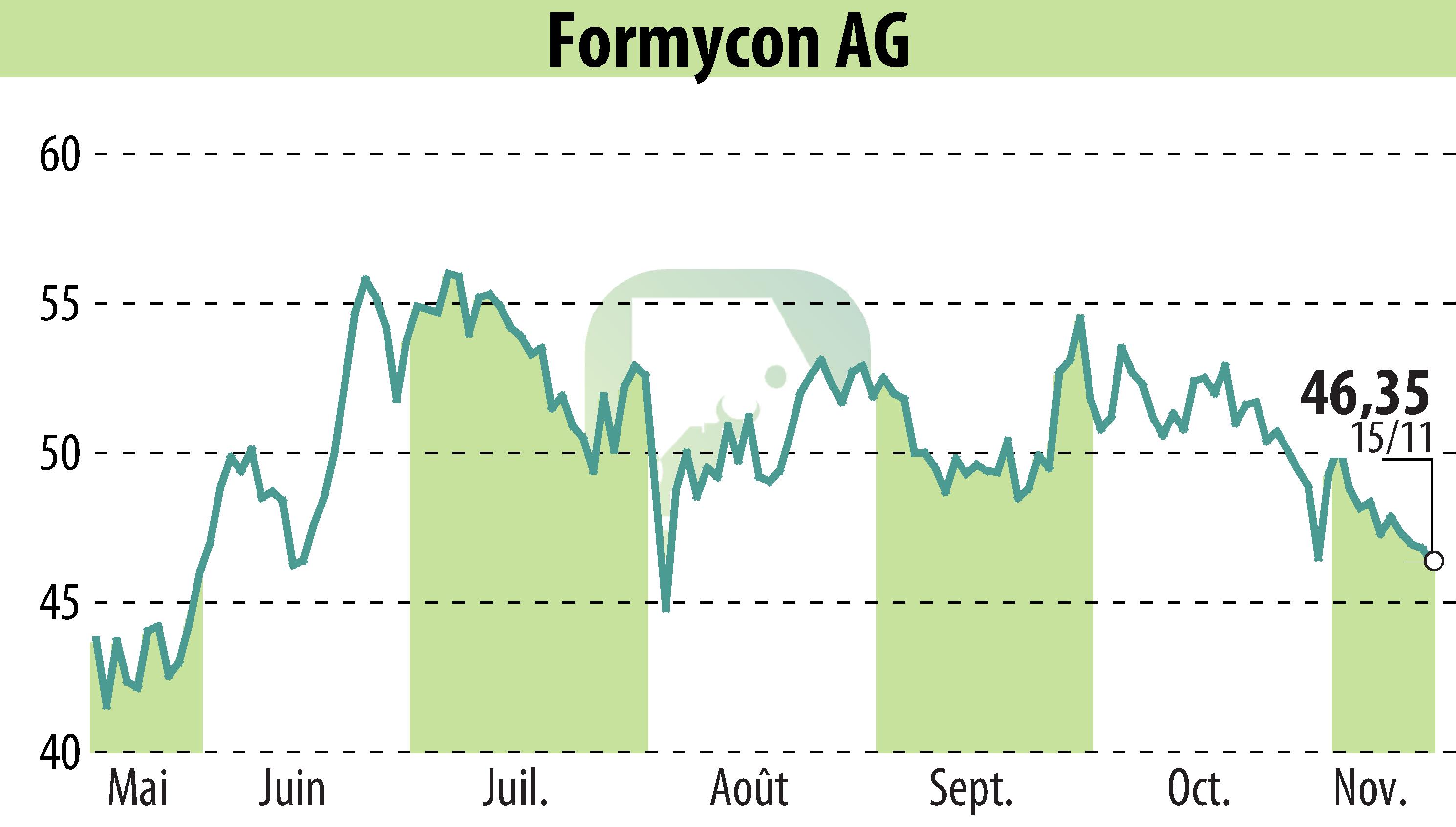
Formycon AG has received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency for FYB203, a biosimilar to Eylea®. This recommendation paves the way for its approval in treating neovascular age-related macular degeneration (nAMD) and other retinal diseases such as diabetic macular edema and myopic choroidal neovascularisation. The decision by the European Commission is anticipated in January 2025.
FYB203, marketed under the tradenames AHZANTIVE® and Baiama®, aims to offer a cost-effective alternative to Eylea®, which generated around $9 billion in sales in 2023. Approved by the U.S. FDA in June, the endorsement marks Formycon's continued efforts to enhance accessibility to effective biosimilar treatments.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
