on GENFIT (EPA:GNFT)
GENFIT: Accelerated approval of Iqirvo® by the FDA for Primary Biliary Cholangitis
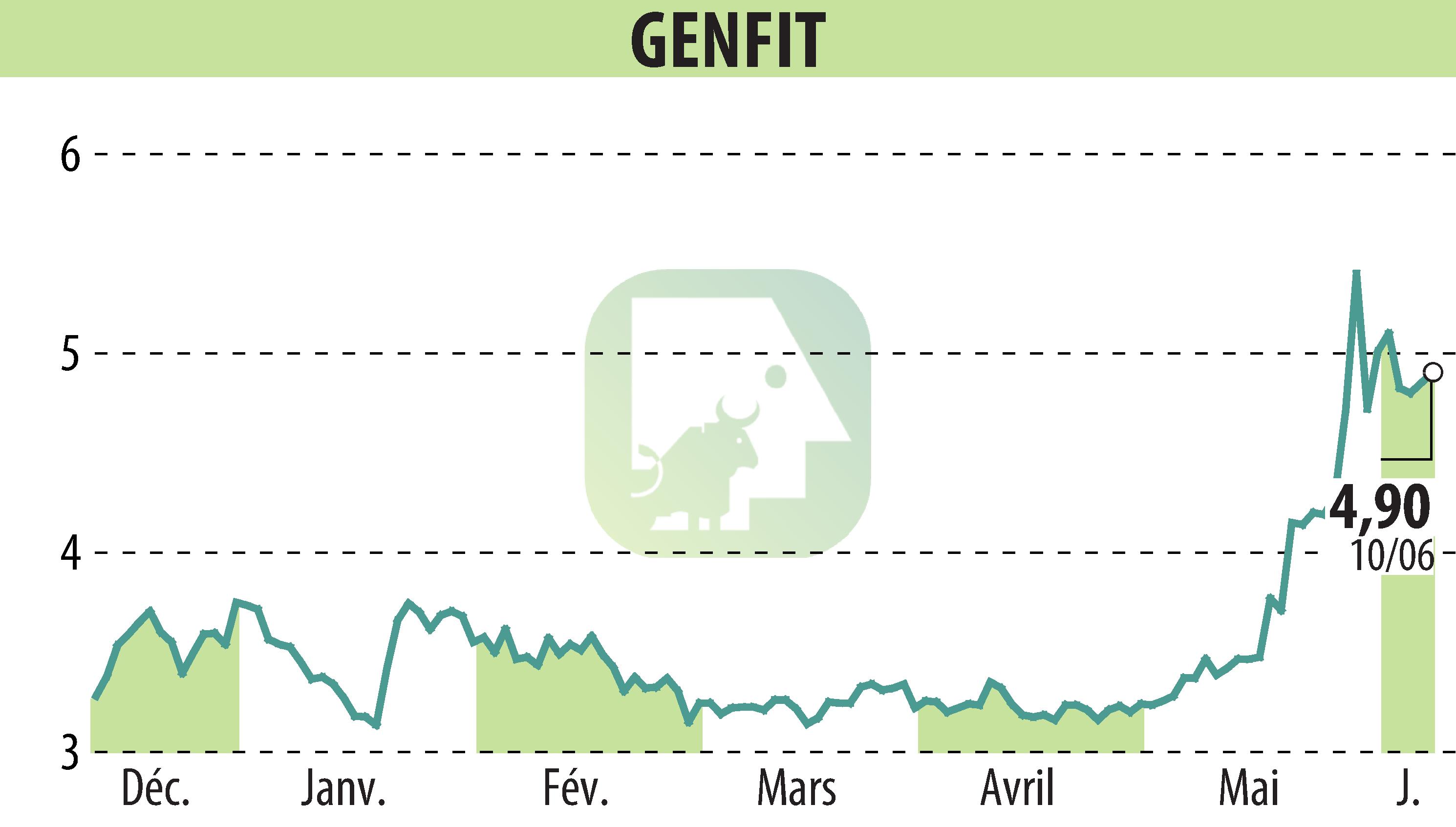
GENFIT, a biopharmaceutical company, has reached a major milestone with the accelerated approval by the US FDA of Iqirvo® (elafibranor) 80 mg. This drug, developed by GENFIT, will be marketed by Ipsen. It is the first treatment for Primary Biliary Cholangitis (PBC) approved by the FDA for adult patients whose response to standard treatment is insufficient or intolerable.
GENFIT will receive a milestone payment of €48.7 million from Ipsen upon the first commercial sale of Iqirvo in the United States, in addition to royalties of up to 20%. The expected revenues will offer GENFIT new financial opportunities for the development of new programs, mainly focused on Acute on Chronic Liver Failure (ACLF).
This achievement marks a new chapter for GENFIT, consolidating its research and development efforts for rare liver diseases. Additional clinical trials are needed to confirm the clinical benefit of Iqirvo, which is essential for its final approval.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENFIT news
