on Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma to Unveil Clinical Data on HDP-101 at EHA 2025
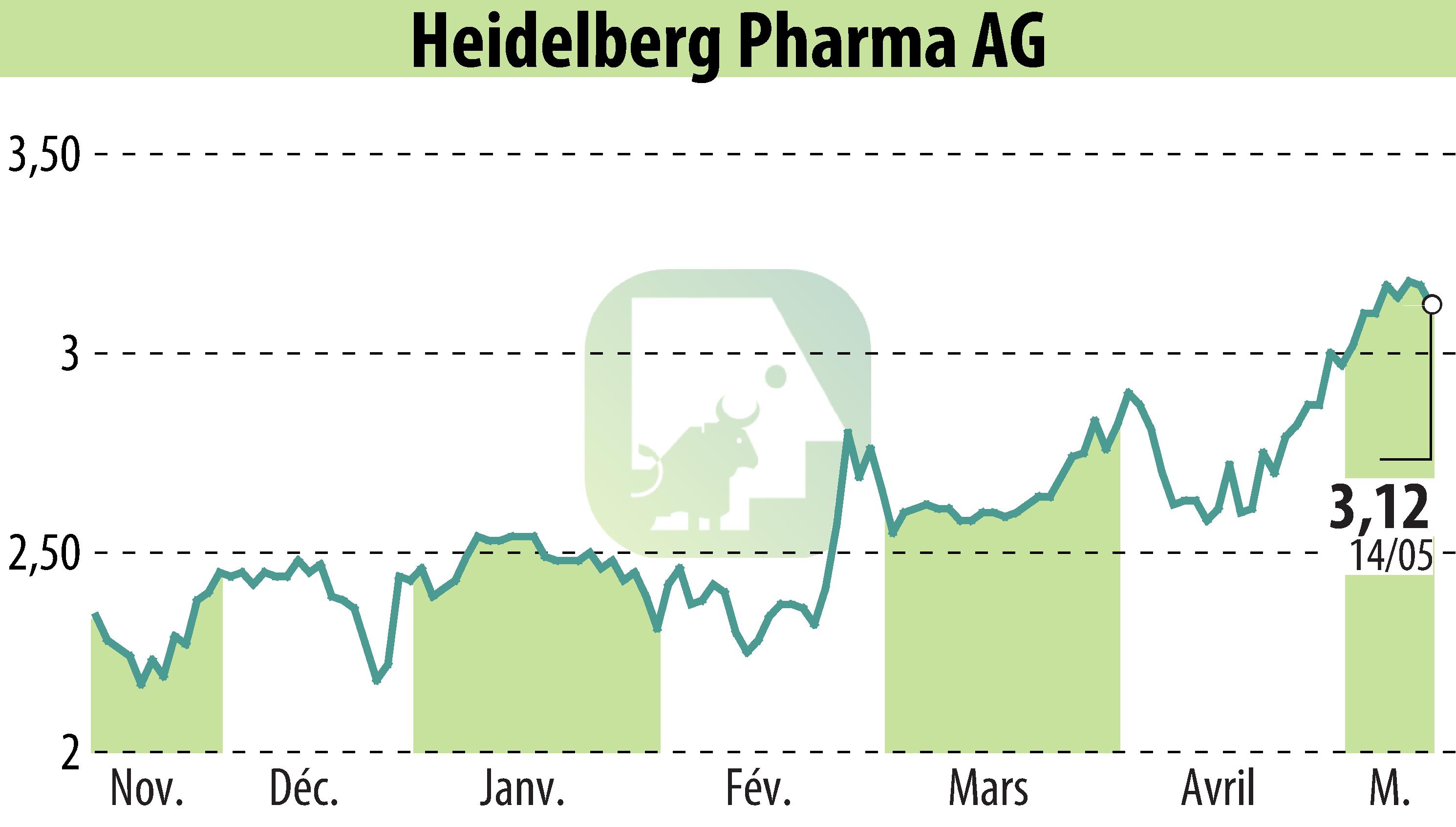
Heidelberg Pharma AG is set to present new clinical data on HDP-101, its lead Amanitin-based Antibody Drug Conjugate (ADC) candidate, at the European Hematology Association Congress in Milan from June 12-15, 2025. This revelation comes as part of the biotech firm's ongoing Phase I/IIa clinical trial for treating relapsed or refractory Multiple Myeloma.
The clinical trial has shown promising results, including a complete response in a female patient who was heavily pretreated. HDP-101 exhibits encouraging biological activity and objective improvements in several patients. The study continues to advance, currently in cohort 8 at a dose of 140 µg/kg.
Following the congress, Heidelberg Pharma will host an R&D webinar on June 17, discussing the ongoing trial with input from Key Opinion Leaders in Multiple Myeloma treatment. This event aims to provide greater insights into HDP-101's potential as a treatment option.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Heidelberg Pharma AG news
