on INNATE PHARMA (EPA:IPH)
Innate Pharma Receives Breakthrough Therapy Designation from FDA
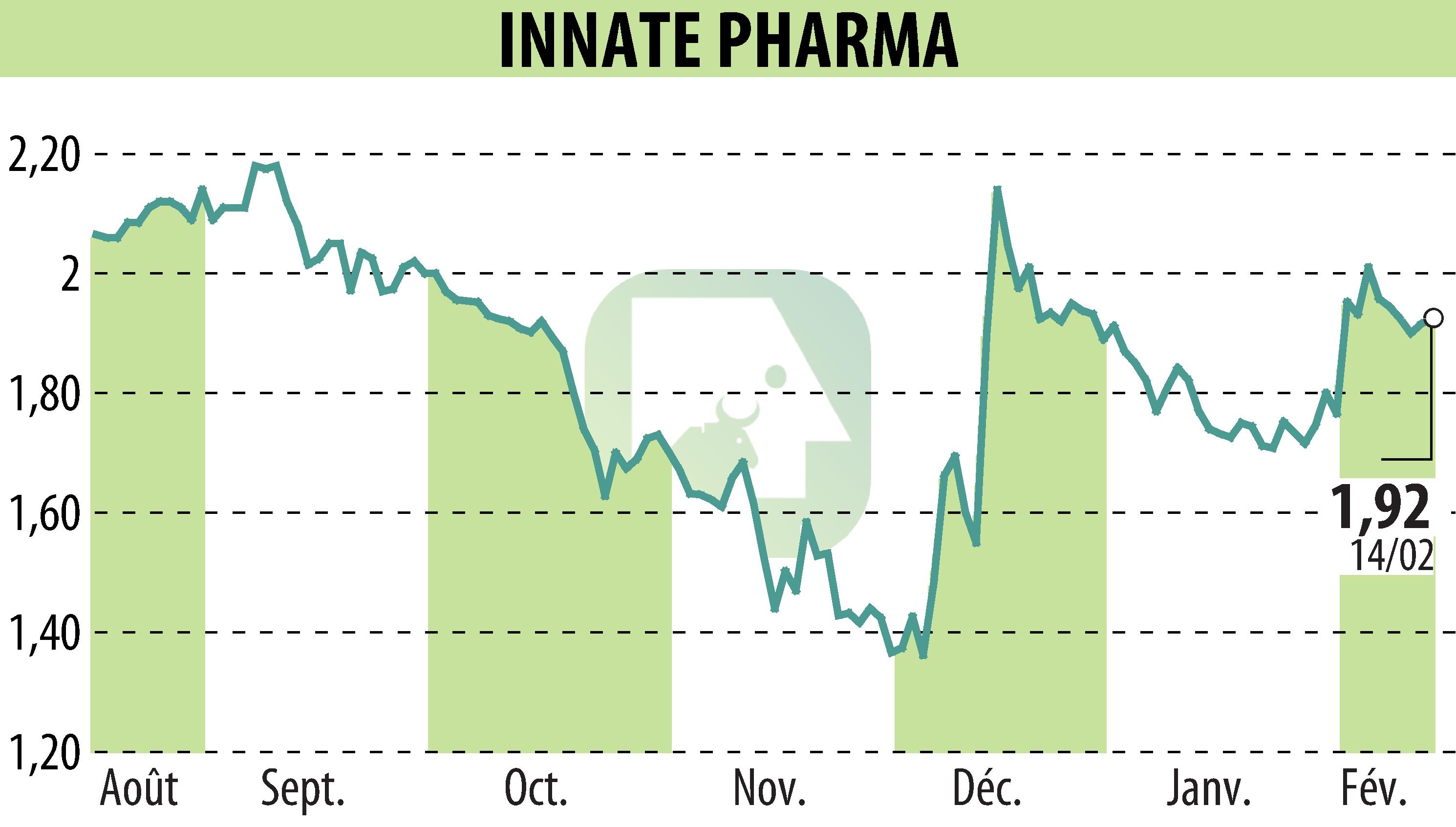
Innate Pharma has received Breakthrough Therapy designation from the FDA for lacutamab, intended for adult patients with relapsed or refractory Sézary syndrome. This designation is based on promising results from the Phase 2 TELLOMAK study, showing significant efficacy and a good safety profile. Breakthrough Therapy accelerates the regulatory development of treatments for serious diseases, adding to the Fast Track and PRIME designations previously received.
The company is in discussions with the authorities for a phase 3 trial on cutaneous T-cell lymphoma and is looking for a partner. Launched in the United States and Europe, lacutamab is focused on a medical market with few effective options available. Its potential to significantly improve on current treatments is recognized. Innate Pharma continues its development to transform the care of patients with Sézary syndrome.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all INNATE PHARMA news
