on NOVACYT (EPA:ALNOV)
IVDR Certification for Yourgene® QST*R Base Diagnostic Test
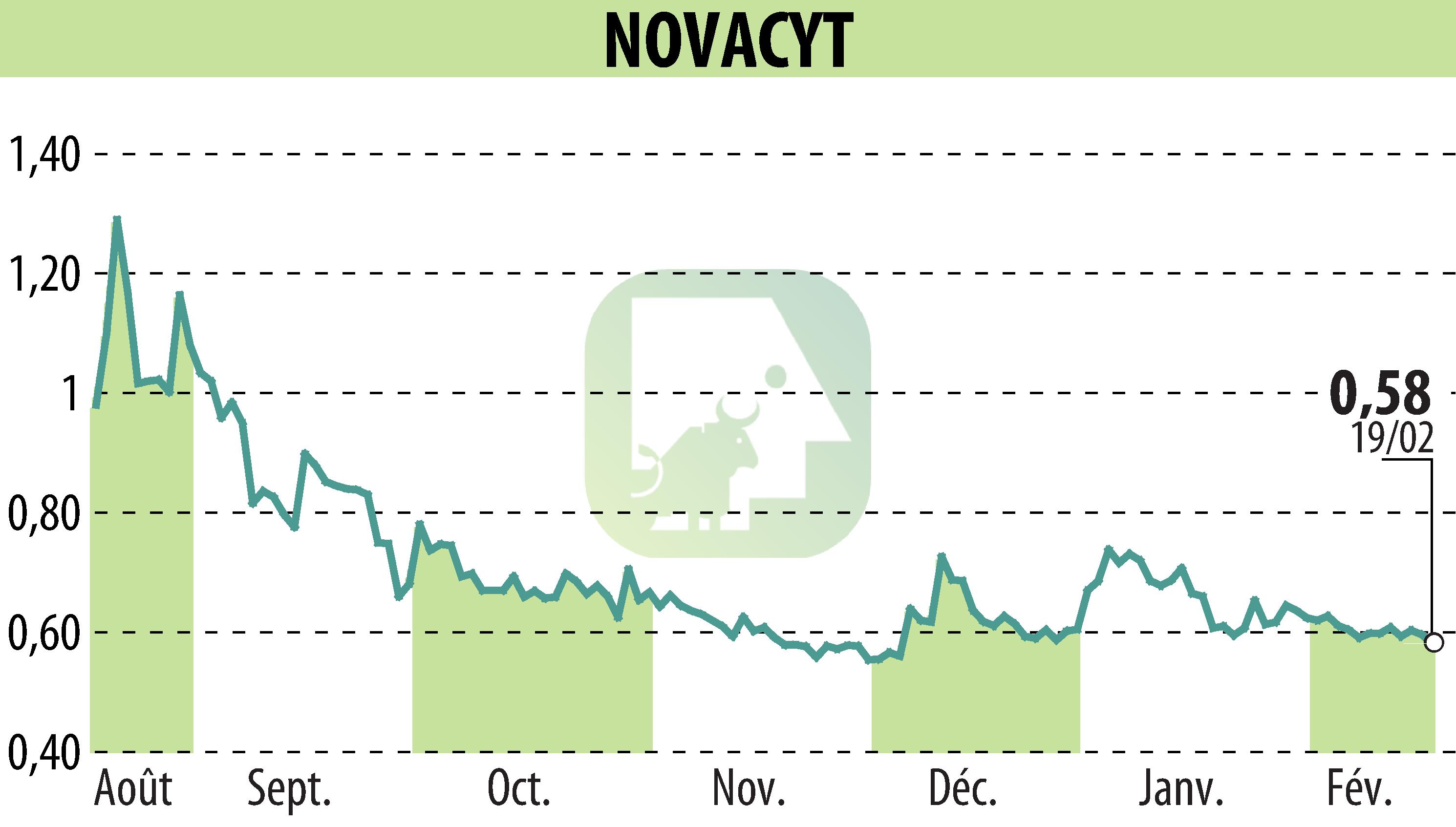
Novacyt, a molecular diagnostics company, has achieved IVDR certification for its Yourgene® QST*R Base test. This in vitro test is intended for healthcare professionals in molecular laboratories to diagnose common autosomal and chromosomal abnormalities during pregnancy.
Yourgene® QST*R Base offers rapid diagnosis of trisomies 21, 18 and 13 using 22 markers. The results are reliable in over 99% of samples. The certification, issued by the BSI, guarantees the test's compliance with the quality, safety and performance standards required by the EU.
This is Novacyt’s third IVDR certification, strengthening its position in the field of medical diagnostics. The test complements the company’s reproductive health offering, adding to NIPT testing.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NOVACYT news
