on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Advances Crofelemer Study in Europe
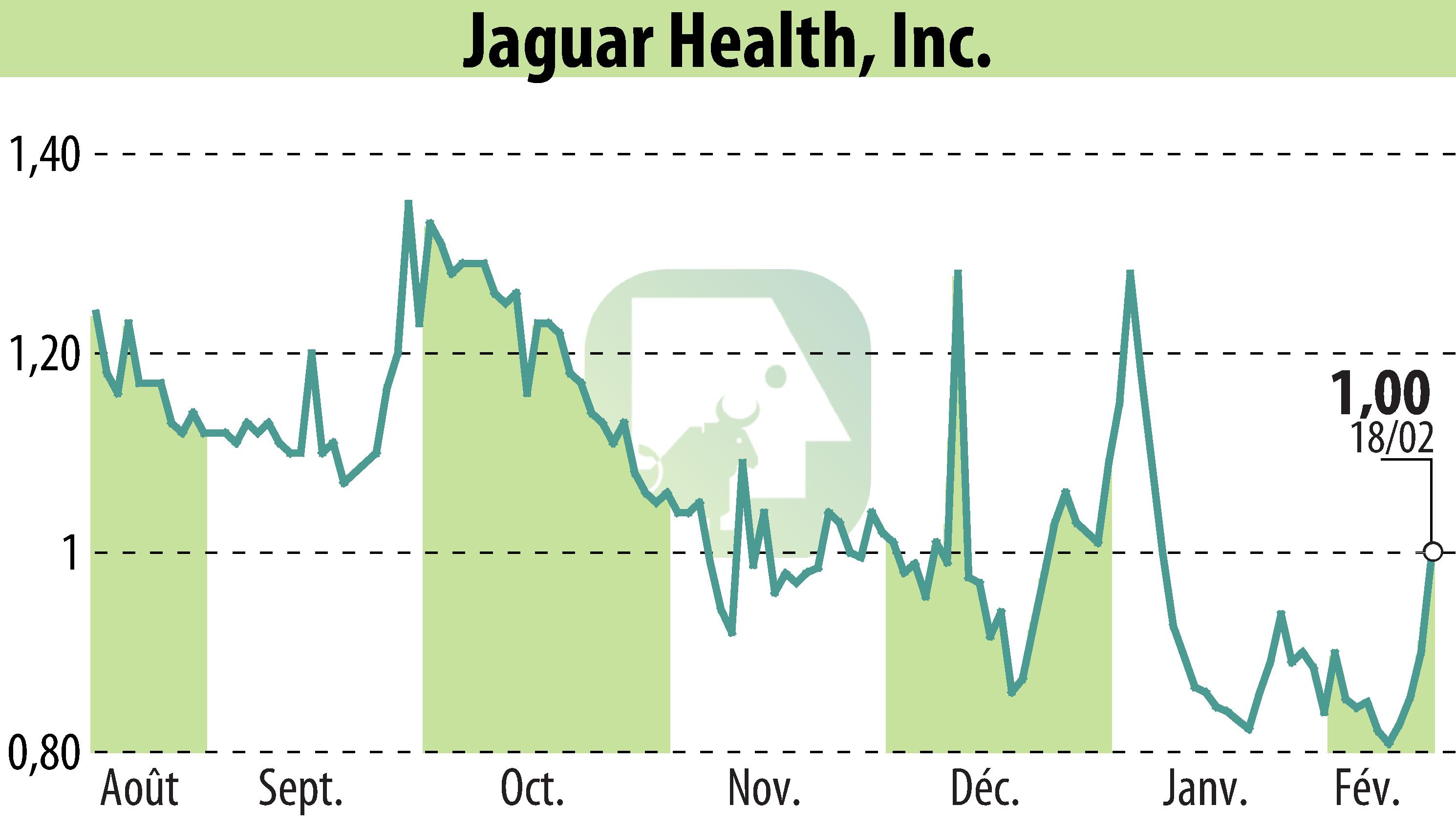
Jaguar Health, Inc. has received regulatory clearance in Germany and Italy to proceed with a Phase 2 study of crofelemer for Short Bowel Syndrome with Intestinal Failure (SBS-IF) in adults. The clinical trial aims to evaluate Jaguar's plant-based prescription drug's efficacy, marking a significant step in rare disease research. Initiated in February, this study is part of a broader effort, including five clinical trials for SBS-IF and Microvillus Inclusion Disease (MVID) in various regions.
With orphan-drug designation by the FDA and EMA for SBS-IF and MVID, crofelemer presents a potential treatment for these severe conditions. Jaguar has also initiated trials for MVID in children and in the UAE for both SBS-IF and MVID. Results from these studies may be expected by 2025, potentially facilitating early patient access to crofelemer through European healthcare guidelines.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
