on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Approves Proposals and Anticipates Key Developments in 2025
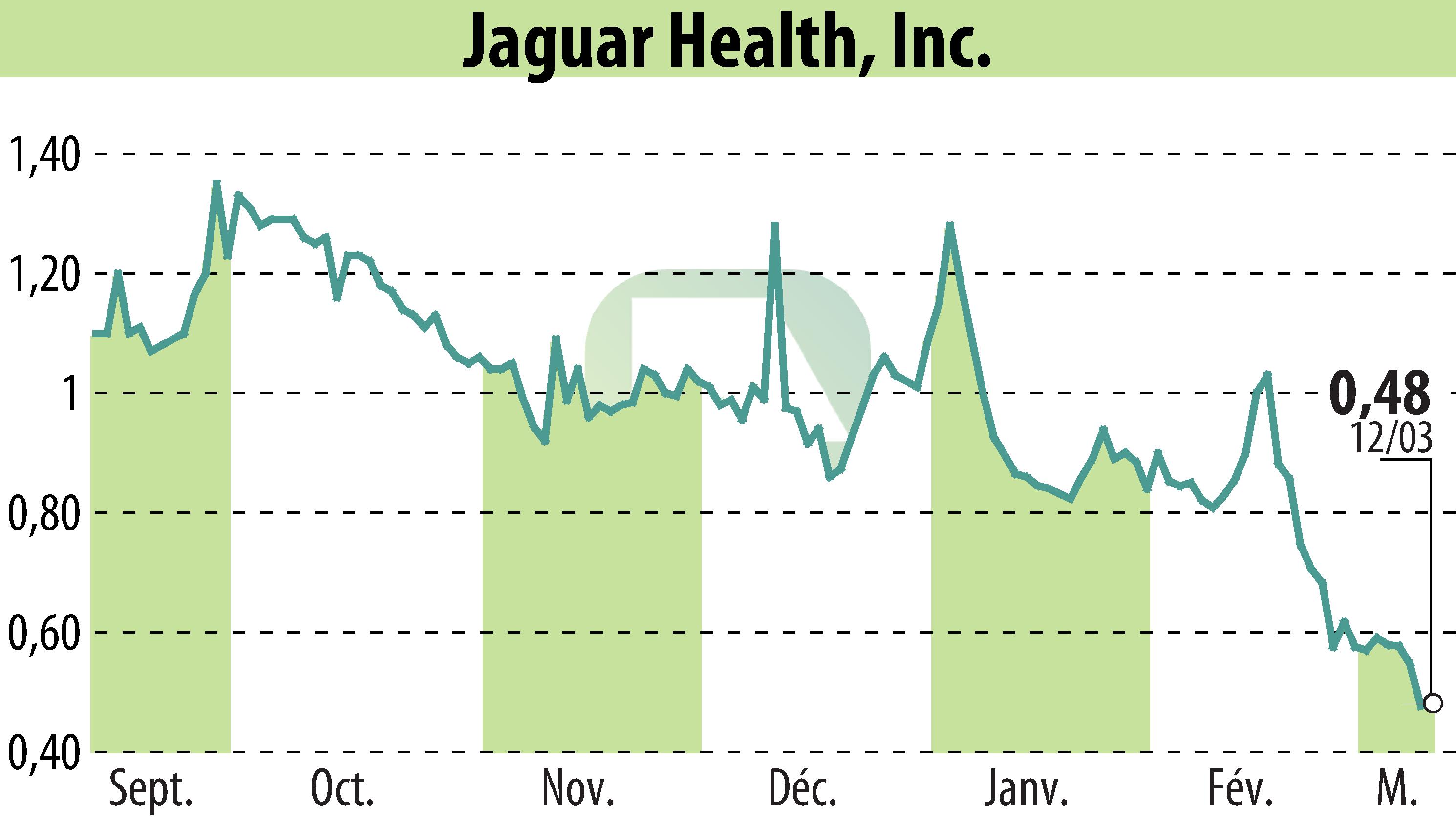
Jaguar Health, Inc. announced the approval of all proposals during its March 2025 Special Meeting of Stockholders. This approval aligns with the company's strategic focus on advancing its plant-based drug, crofelemer, in treating specific rare diseases and cancer therapy-related diarrhea.
Results from proof-of-concept trials for crofelemer targeting short bowel syndrome with intestinal failure (SBS-IF) and microvillus inclusion disease (MVID) are expected by Q2 2025. These findings could facilitate early patient access in the EU and potentially lead to regulatory approvals in the US and elsewhere.
An FDA meeting is also anticipated in Q2 2025 to discuss significant Phase 3 trial results of crofelemer for breast cancer-related diarrhea. Jaguar remains committed to efficient regulatory pathways for crofelemer's broader availability.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
