on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Launches Phase 2 Study for Microvillus Inclusion Disease
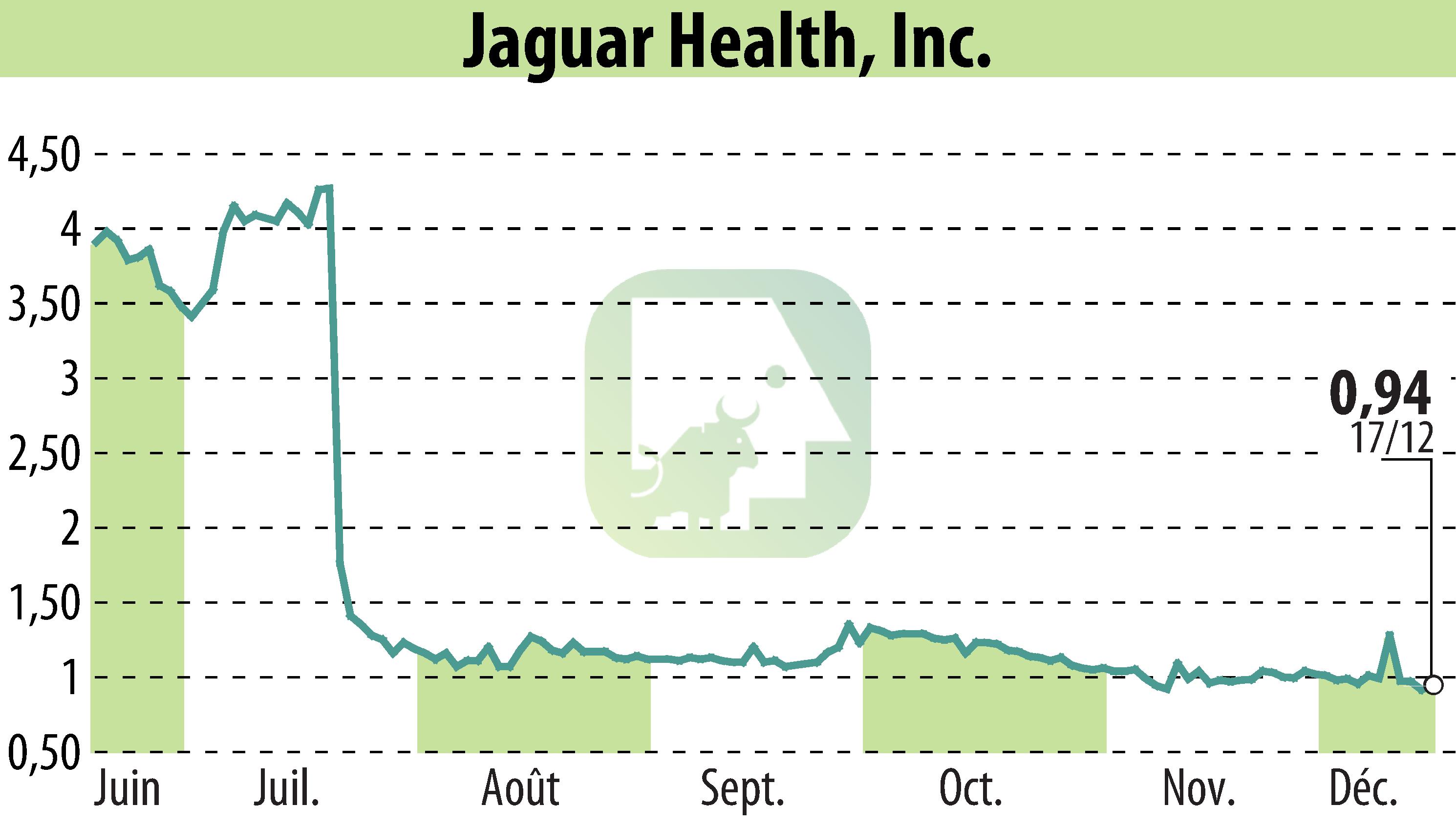
Jaguar Health, Inc. has initiated a Phase 2 study to evaluate the efficacy of crofelemer, a plant-based prescription drug, for Microvillus Inclusion Disease (MVID) in pediatric patients. Crofelemer has received Orphan-Drug Designation from the FDA and European Medicines Agency. The study, involving double-blind, placebo-controlled trials, marks a significant step for Jaguar's clinical efforts in rare diseases.
Lisa Conte, Jaguar's CEO, emphasized the importance of this milestone. The study is part of a broader initiative encompassing three proof-of-concept trials and two Phase 2 studies targeting rare conditions like MVID and short bowel syndrome with intestinal failure.
MVID, characterized by severe diarrhea and malabsorption, affects a small global population. Currently, there are no approved drug treatments for this condition. Jaguar hopes positive outcomes could lead to U.S. approval and expanded patient access in certain EU countries.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
