on Moderna, Inc. (NASDAQ:MRNA)
Japan Approves Updated Moderna COVID-19 Vaccine Targeting SARS-CoV-2 Variant JN.1
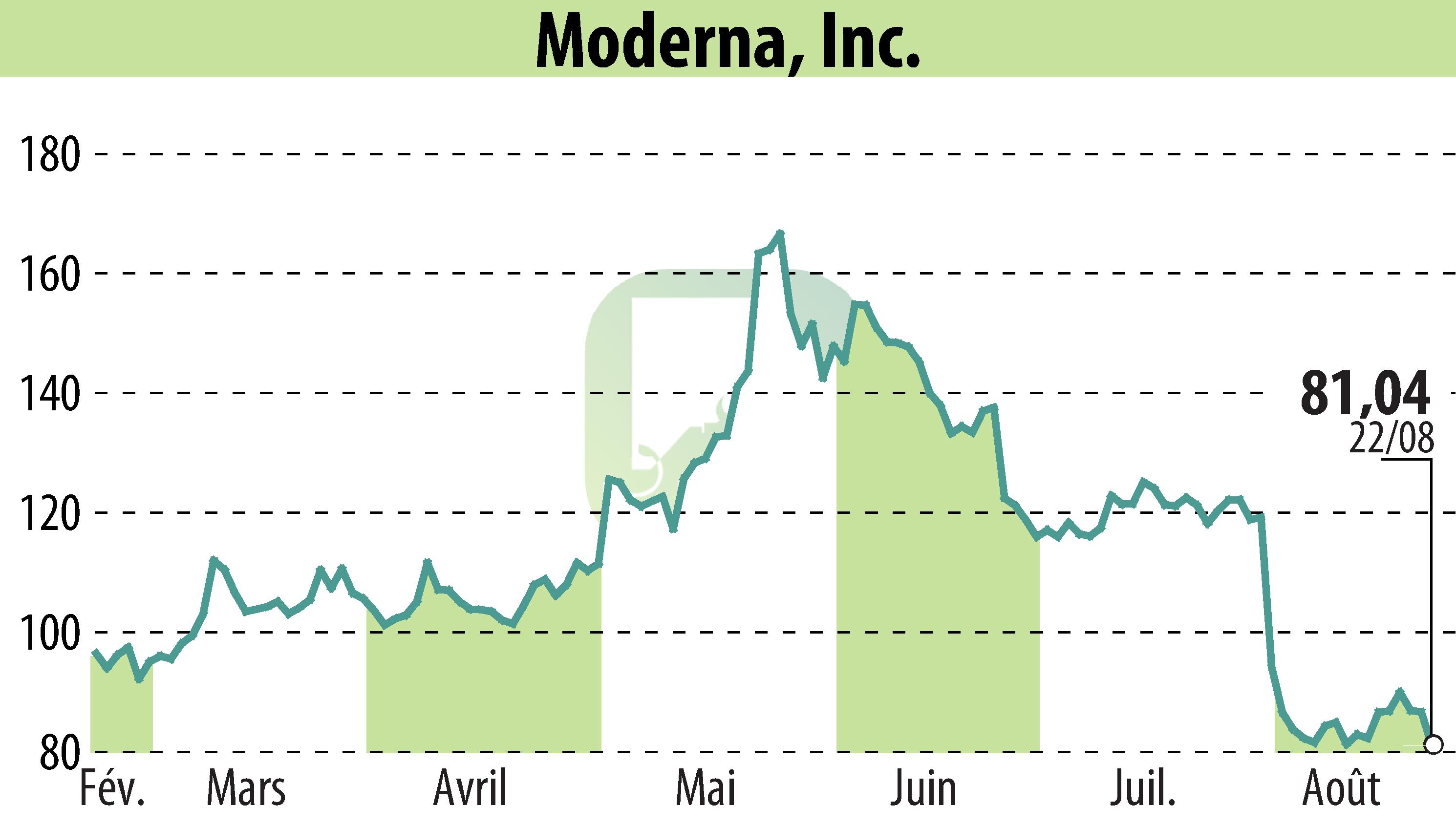
CAMBRIDGE, MA / ACCESSWIRE / August 23, 2024 - Moderna, Inc. (NASDAQ:MRNA) has received approval from Japan's Ministry of Health, Labour and Welfare (MHLW) for a partially changed application of its COVID-19 mRNA vaccine, Spikevax, tailored to target the SARS-CoV-2 variant JN.1.
"We appreciate the MHLW's approval," stated Stéphane Bancel, CEO of Moderna. "It's vital that people are vaccinated with the latest vaccines to guard against circulating strains. With COVID-19 vaccinations becoming routine, individuals can get their updated COVID-19 and flu vaccines together this fall."
In May 2024, a Japanese health ministry panel recommended updating COVID-19 vaccines to target the JN.1 variant for the 2024/2025 national immunization program. Japan's NIP will offer COVID-19 vaccination to individuals aged 65 and older and those aged 60 to 64 with qualifying health conditions.
Moderna has also partnered with Mitsubishi Tanabe Pharma Corporation to co-promote its mRNA vaccine portfolio in Japan, including Spikevax.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
