on MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Initiates Ketamir-2 Clinical Trials
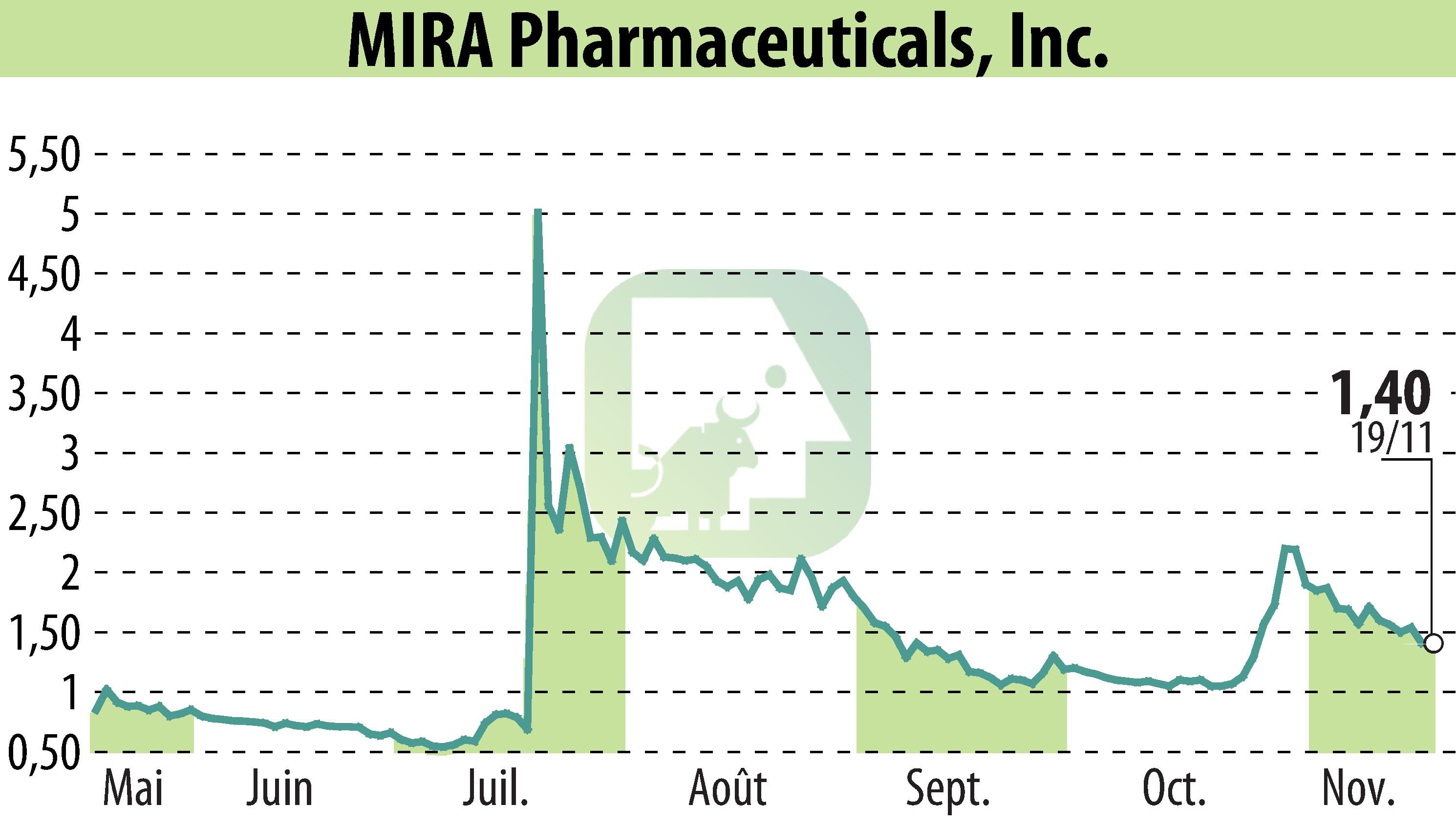
MIRA Pharmaceuticals has announced the commencement of its Phase I/IIa clinical trials for Ketamir-2, a novel oral ketamine analog, at The Centre for Human Drug Research in Leiden, The Netherlands. Initial trials focusing on safety and efficacy are expected to begin in early 2025, targeting healthy subjects first, followed by diabetic neuropathy patients later that year.
CHDR's PainCart® technology will be used to assess pain response, offering early efficacy data. The trial also includes separate psychoactivity evaluations. The study is designed to yield early proof-of-concept data crucial for advancing Ketamir-2's development.
MIRA remains in a strong financial position, aiming for strategic partnerships and exploring broader applications of Ketamir-2. The drug has outperformed existing treatments like pregabalin and gabapentin in preclinical trials, showing promise as a safe, non-addictive treatment for pain and depression.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MIRA Pharmaceuticals news
