on MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Receives FDA Green Light for Ketamir-2 Trials
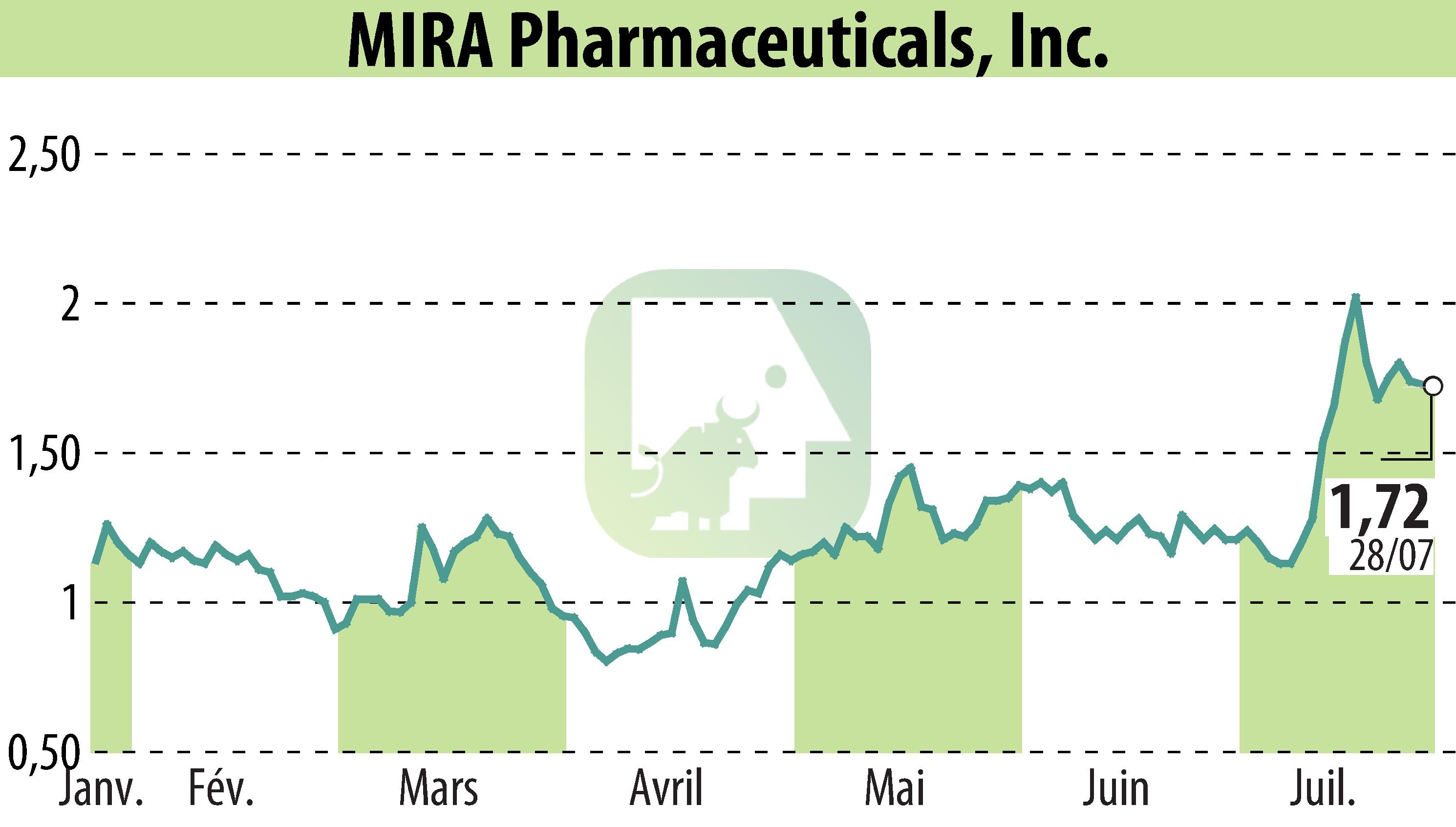
MIRA Pharmaceuticals has announced FDA clearance for its Investigational New Drug application for Ketamir-2. This novel oral NMDA receptor antagonist is developed for treating neuropathic pain. Preclinical studies on Ketamir-2 showed no adverse CNS effects and superior efficacy to gabapentin and pregabalin.
MIRA is completing the Single Ascending Dose portion of its Phase 1 trial and plans to initiate a Phase 2a study in the U.S. by the end of 2025. The company is also considering trials in diabetic and chemotherapy-induced peripheral neuropathy, reflecting a strategic expansion into these areas.
With an increasing prevalence of neuropathic pain in North America, Ketamir-2's non-opioid profile may address access barriers associated with current therapies. MIRA's progression with Ketamir-2 and its potential merger with SKNY Pharmaceuticals underline its strategic developments in the pharmaceutical landscape.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MIRA Pharmaceuticals news
