on Moderna, Inc. (NASDAQ:MRNA)
Moderna Announces Positive Phase 3 Data for Combination Vaccine Against Influenza and COVID-19
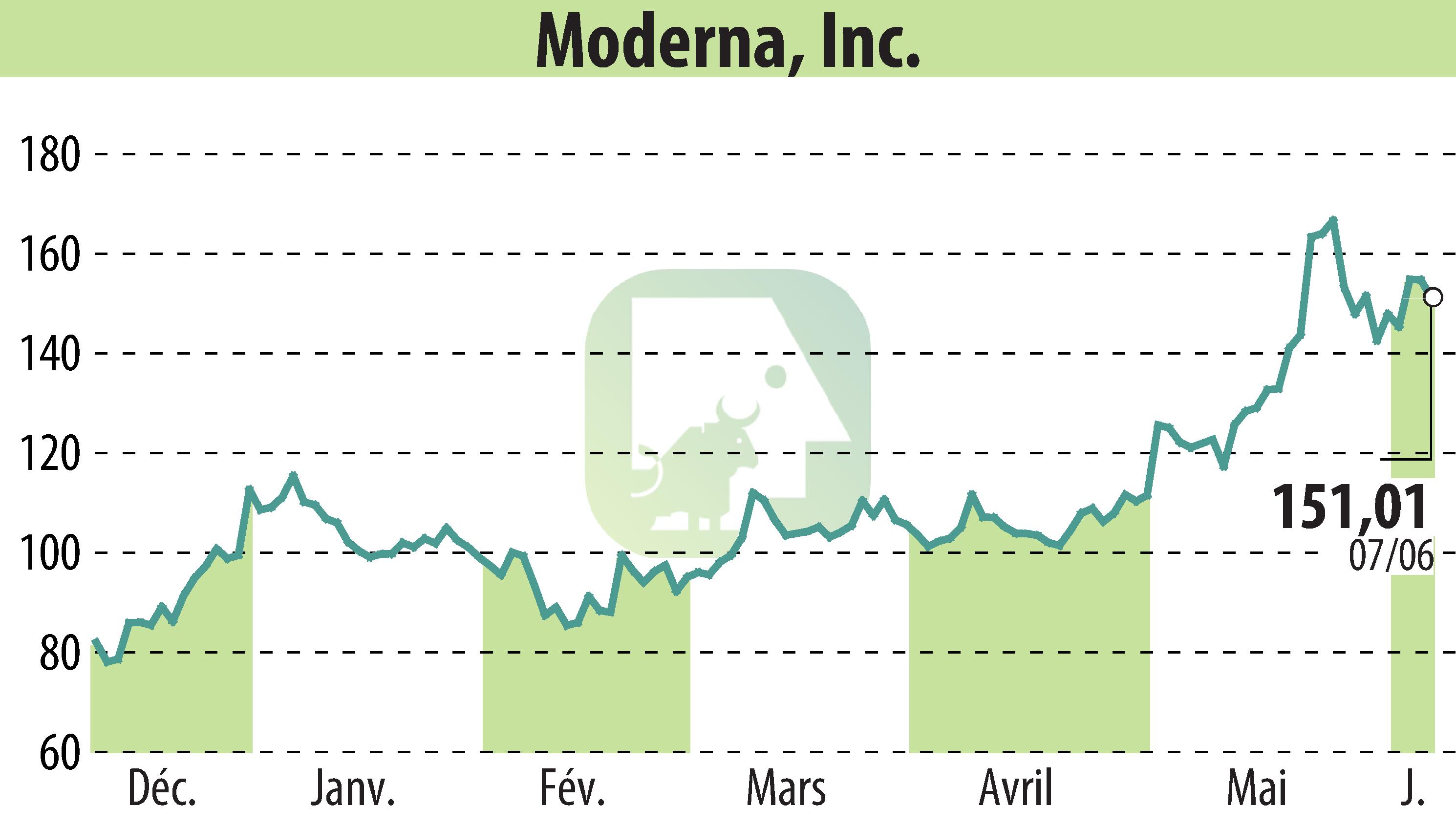
Moderna, Inc. (NASDAQ:MRNA) has announced that its Phase 3 trial of mRNA-1083, a combination vaccine against influenza and COVID-19, met its primary endpoints. The vaccine generated higher immune responses than the licensed flu and COVID vaccines for adults aged 50 and older.
CEO Stéphane Bancel highlighted the potential of combination vaccines to reduce the burden on health systems and offer more convenient vaccination options. Moderna’s mRNA-1083 includes components of mRNA-1010 for influenza and mRNA-1283 for COVID-19, both showing positive Phase 3 results independently.
The Phase 3 trial, listed under ClinicalTrials.gov Identifier:NCT06097273, involved around 8,000 adults in two age cohorts. mRNA-1083 demonstrated superior immune responses compared to Fluzone HD® and Spikevax® in the 65+ age group and Fluarix® and Spikevax® in the 50-64 age group.
The vaccine showed a favorable safety profile, with most adverse reactions being mild. Moderna plans to present the data at an upcoming medical conference and engage with regulators.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
