on Moderna, Inc. (NASDAQ:MRNA)
Moderna Doses First Participant in Phase 3 Trial of Norovirus Vaccine
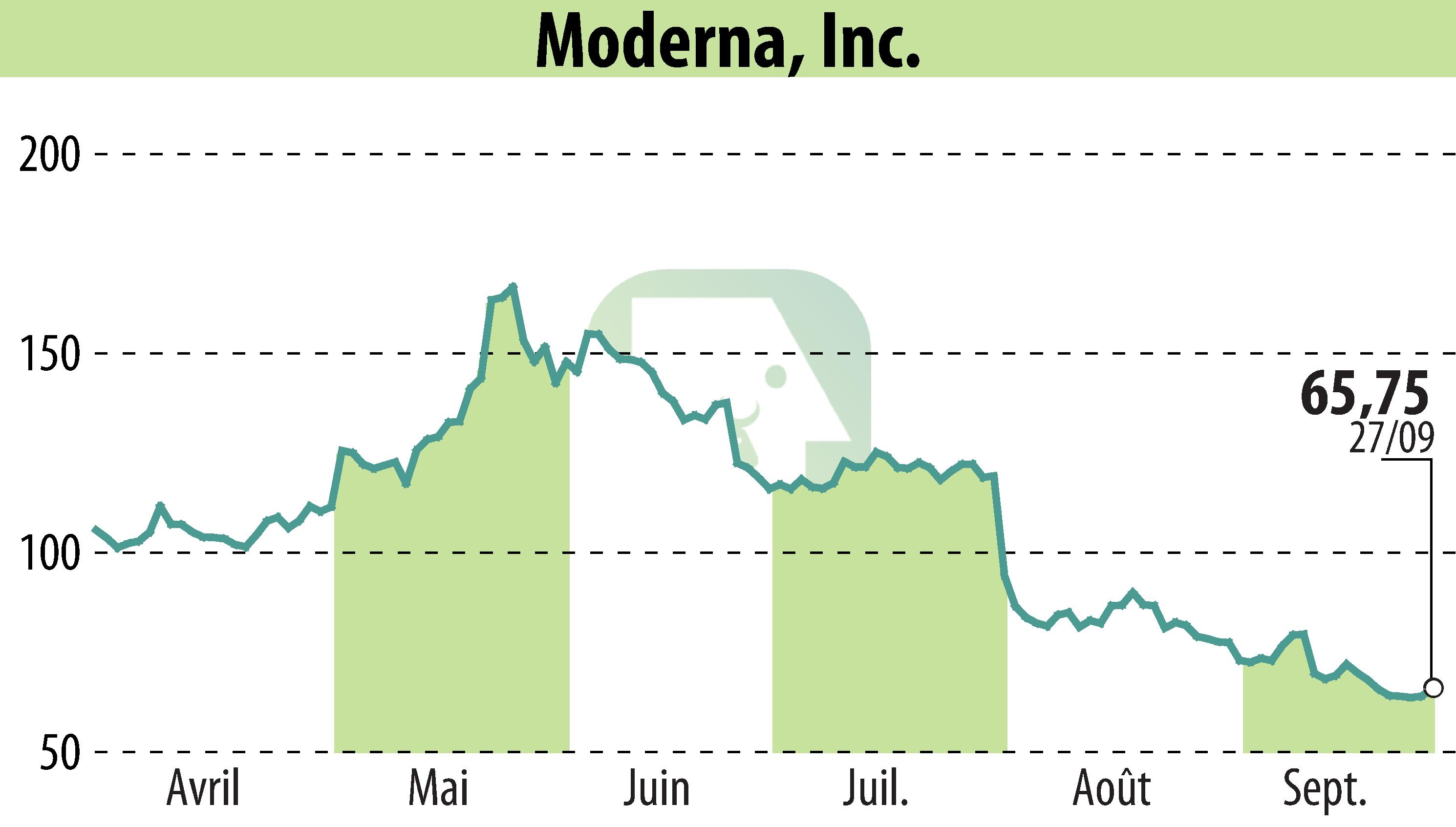
CAMBRIDGE, MA / ACCESSWIRE / September 30, 2024 / Moderna, Inc. (NASDAQ:MRNA) announced the commencement of the pivotal Nova 301 Phase 3 trial for its investigational norovirus vaccine, mRNA-1403. The first U.S. participant has been dosed, with global recruitment underway.
This randomized, observer-blind, placebo-controlled trial will assess the efficacy, safety, and immunogenicity of the mRNA-1403 vaccine. It aims to enroll 25,000 participants, focusing on adults aged 18 and older, with particular attention to those over 60 who are at higher risk for severe norovirus-related health outcomes.
Norovirus is a major public health issue, causing significant morbidity and mortality worldwide, especially among young children and older adults. Approximately 200,000 annual deaths are attributed to norovirus, underscoring the need for effective preventive measures.
mRNA-1403 is designed to protect against multiple norovirus genotypes. The successful delivery of this vaccine would be a critical tool in reducing the global burden of norovirus-related gastroenteritis.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
