on Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives FDA Approval for Updated COVID-19 Vaccine Targeting KP.2 Variant
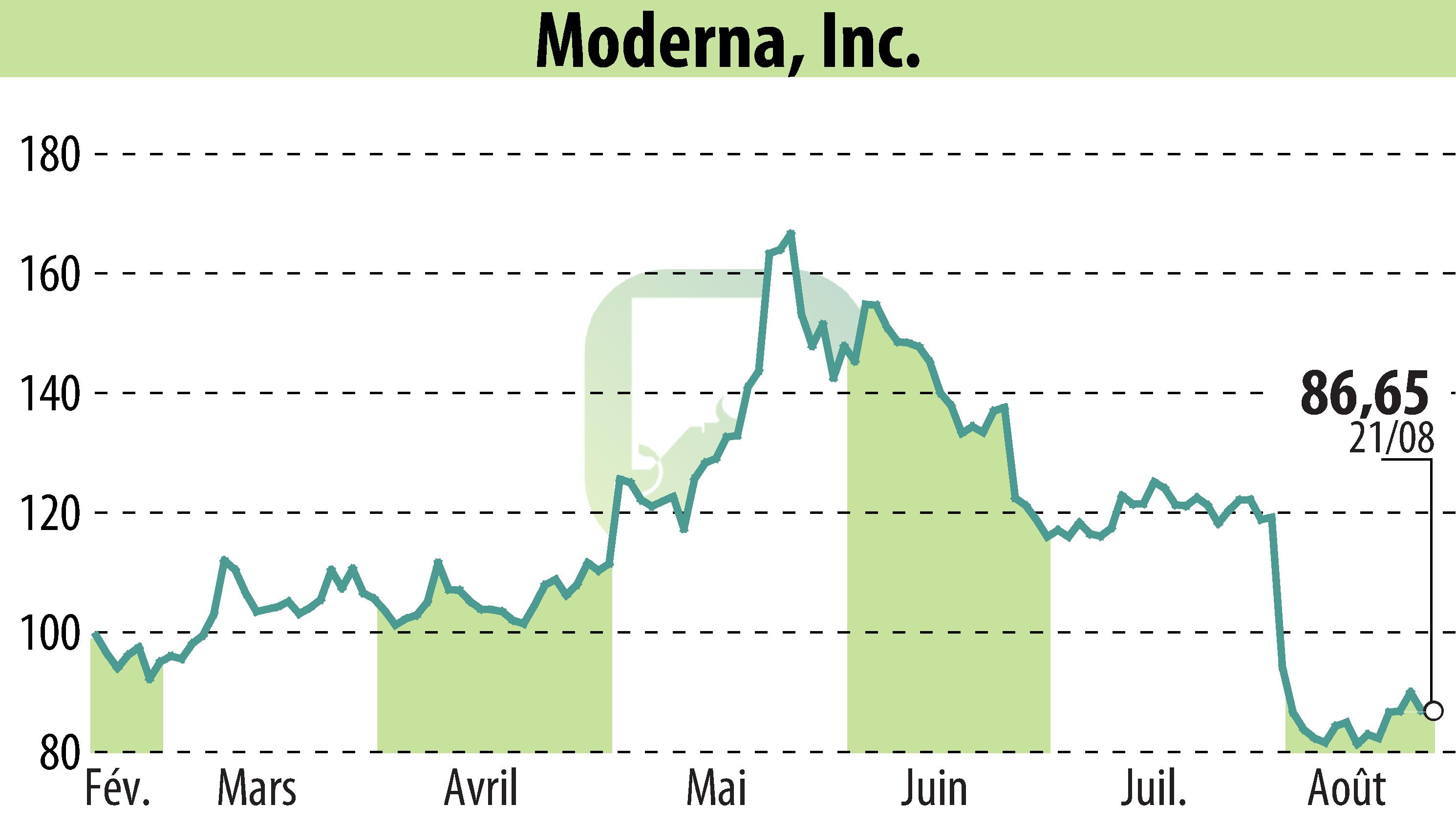
CAMBRIDGE, MA / ACCESSWIRE / August 22, 2024 - Moderna, Inc. (NASDAQ:MRNA) has announced that the U.S. FDA approved the supplemental Biologics License Application for Spikevax® (2024-2025 formula) for individuals aged 12 and above. Emergency Use Authorization has been granted for those aged 6 months through 11 years.
The vaccine targets the KP.2 variant of SARS-CoV-2 and will be available in pharmacies and healthcare settings across the U.S. in the coming days. Moderna CEO Stéphane Bancel emphasized the importance of staying current with COVID-19 vaccinations to prevent severe illness.
The FDA's decision is based on preclinical data and previous evidence supporting the vaccine's efficacy and safety. Moderna's updated vaccine contains a monovalent KP.2 composition as recommended by the FDA.
In addition to the KP.2 variant, Moderna is also developing a vaccine targeting the JN.1 variant, with regulatory decisions anticipated soon.
Moderna's vaccines have been well-tolerated, with common adverse reactions being injection site pain, headache, fatigue, myalgia, and chills.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
