on Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives Health Canada Approval for Updated COVID-19 Vaccine Targeting KP.2 Variant
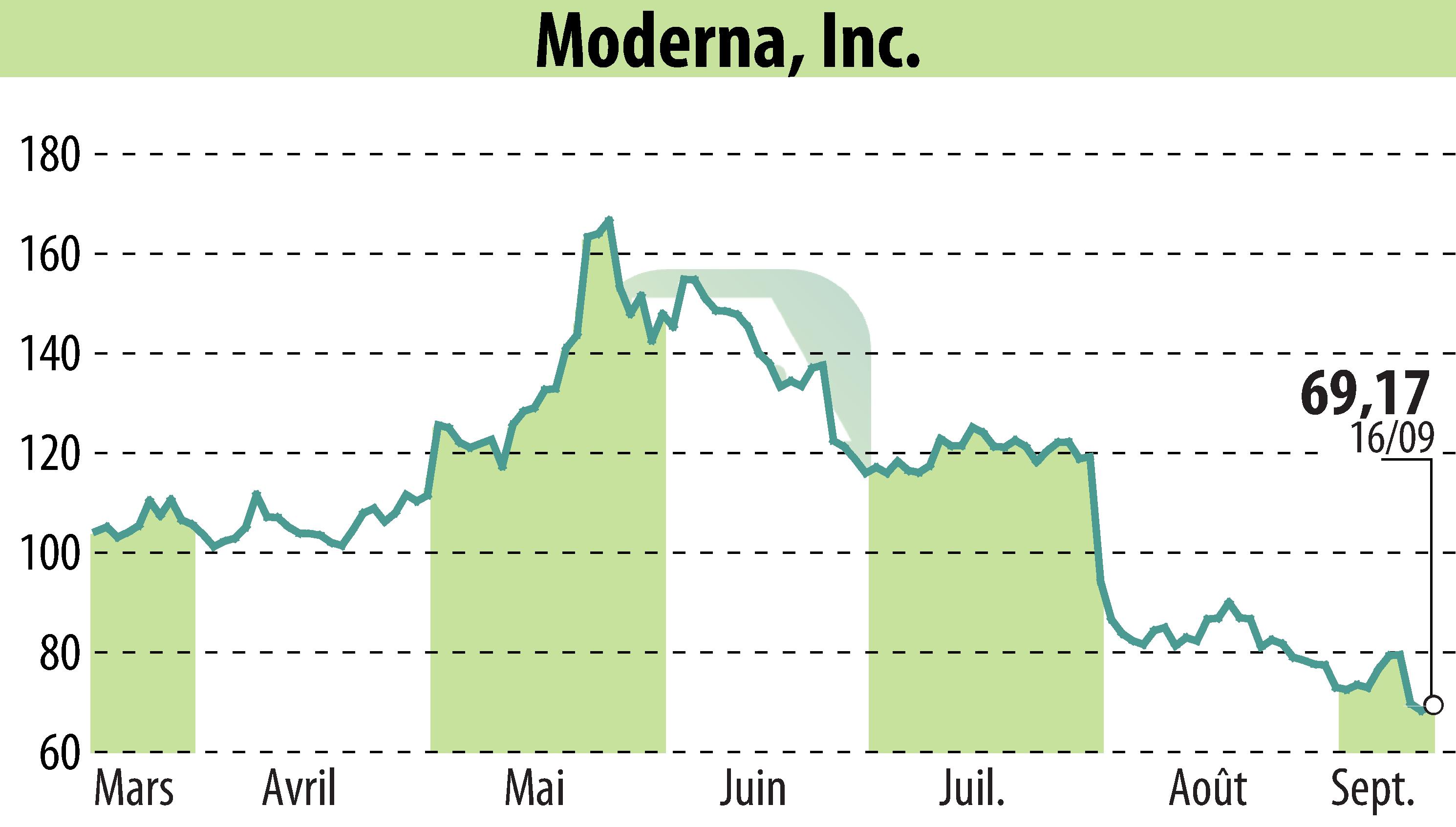
Moderna Inc. announced that Health Canada has authorized its updated COVID-19 vaccine, SPIKEVAX KP.2, for individuals aged six months and older. This vaccine targets the KP.2 sub-lineage of SARS-CoV-2 and is the first updated COVID-19 vaccine approved in Canada for the 2024-2025 season.
With this approval, Moderna will promptly begin delivering the updated vaccine to the Public Health Agency of Canada to ensure timely availability for provincial and territorial vaccination campaigns. The single presentation of SPIKEVAX will allow both adult and pediatric doses to be drawn from the same multidose vial.
Stéphane Bancel, CEO of Moderna, stated, "The authorization of our updated COVID-19 vaccine ensures that Canadians have timely access to the latest vaccines." Dr. Shehzad Iqbal, Moderna Canada's Country Medical Director, encouraged Canadians to discuss updating their COVID-19 vaccinations, along with flu shots, with their healthcare providers.
The approval is based on a combination of manufacturing and pre-clinical data, along with prior clinical and real-world evidence supporting the efficacy and safety of Moderna's mRNA vaccines.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
