on Moderna, Inc. (NASDAQ:MRNA)
Moderna's Investigational Therapeutic for Methylmalonic Acidemia Selected by FDA for START Pilot Program
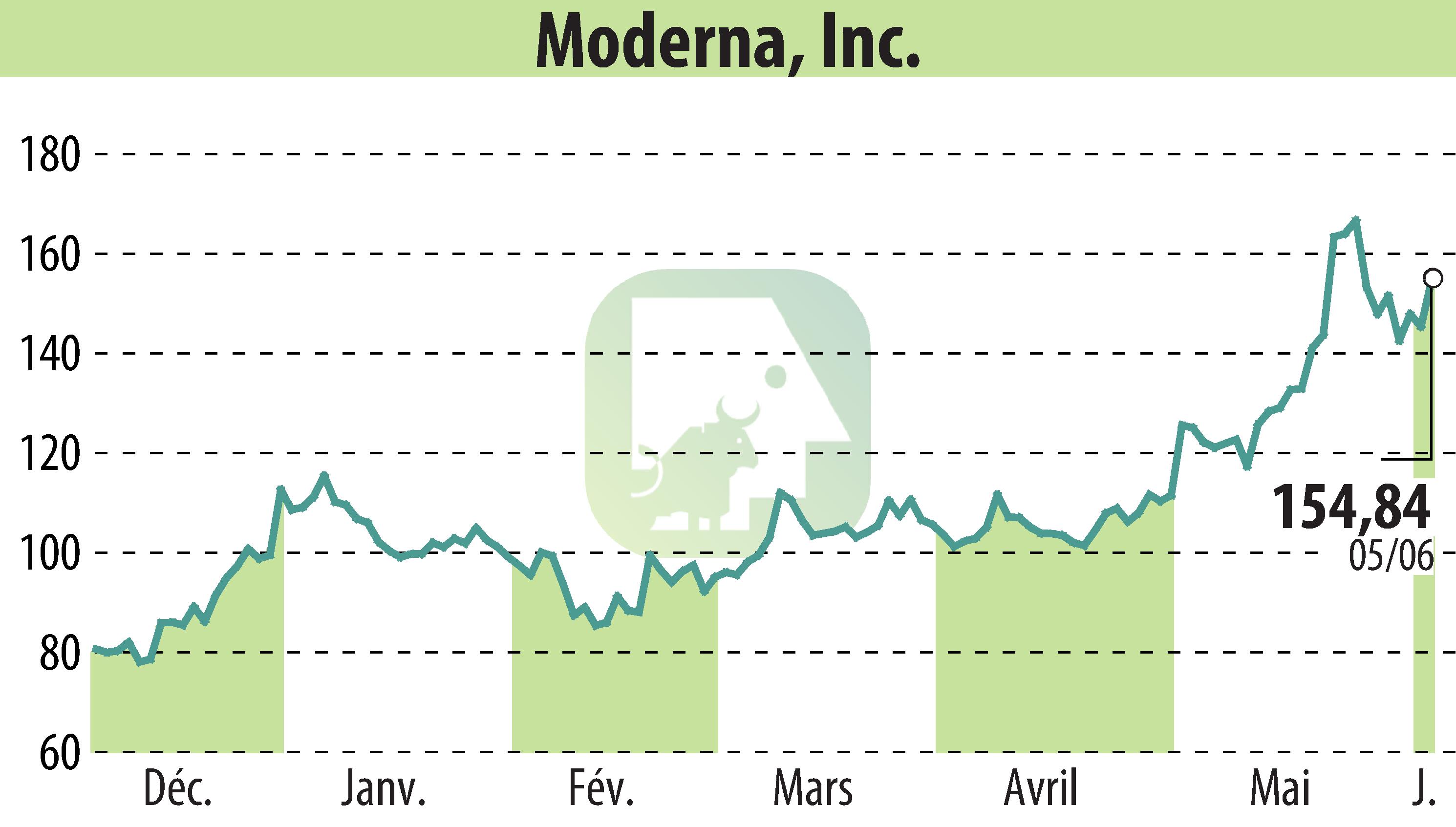
Moderna, Inc. (NASDAQ:MRNA) announced that the FDA has selected mRNA-3705 for the START pilot program. This investigational therapeutic addresses methylmalonic acidemia (MMA) due to methylmalonic-CoA mutase deficiency.
Kyle Holen, M.D., Moderna's Senior VP, highlighted the significance of this selection, noting its potential impact on serious unmet medical needs. The START program aims to accelerate the development of treatments for rare diseases by enhancing FDA-manufacturer communication.
MMA is a rare, life-threatening metabolic disorder caused by a deficiency in the mitochondrial enzyme MUT. Current treatments are limited to dietary measures and organ transplants.
mRNA-3705 is under evaluation in a Phase 1/2 study to assess its safety and tolerability. This program benefits from accelerated interactions with the FDA, aiding the progression toward pivotal clinical studies.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
