on MONOGRAM ORTHOPAEDICS INC (NASDAQ:MGRM)
Monogram Orthopaedics Expands Global Clinical Trials for mBôs TKA System
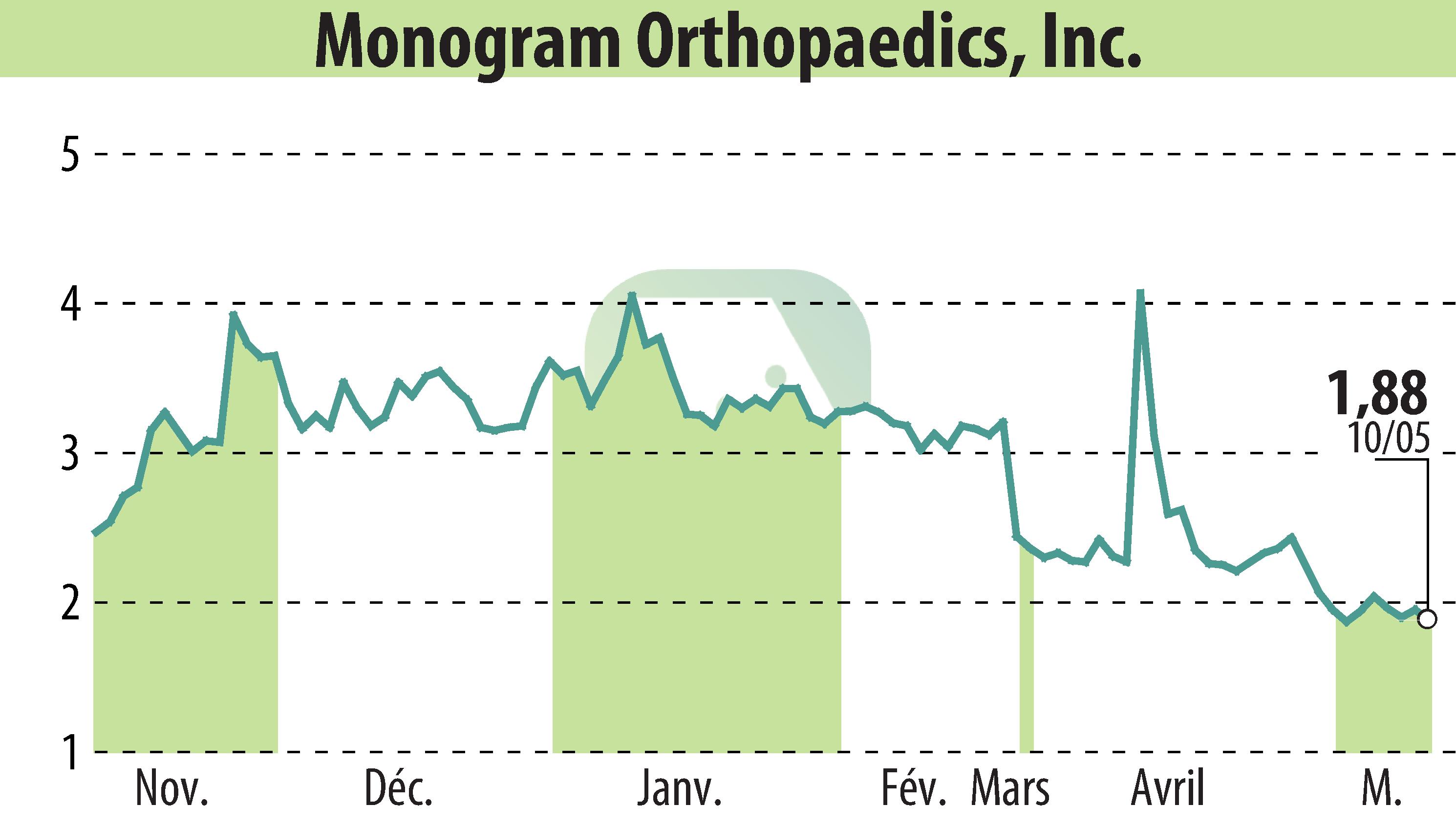
Monogram Orthopaedics Inc., based in Austin, Texas, announced its partnership with a global Contract Research Organization (CRO) to manage its mBôs Total Knee Arthroplasty (TKA) System's clinical trials outside the U.S. This non-disclosed CRO has a track record of successful FDA submissions and will submit the trial results to local regulators for approval.
According to Monogram's CEO, Ben Sexson, this collaboration aims to accelerate the next-generation mBôs system's development and expects complete verification and validation by the first half of 2024. Estimated to cut down significant costs and time, Monogram foresees conducting about 100 knee surgeries across three sites with the cemented version of their FDA-cleared mPress TKA implant.
The clinical trial will abide by FDA and international good clinical practice guidelines, ensuring adherence to all regulatory standards. This trial is designed to primarily collect safety data but will also secure additional data for post-launch marketing and international commercialization.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MONOGRAM ORTHOPAEDICS INC news
