on Nanohale AG (isin : DE000A1EWVY8)
MS Pharma to Commercialize FYB202 in MENA
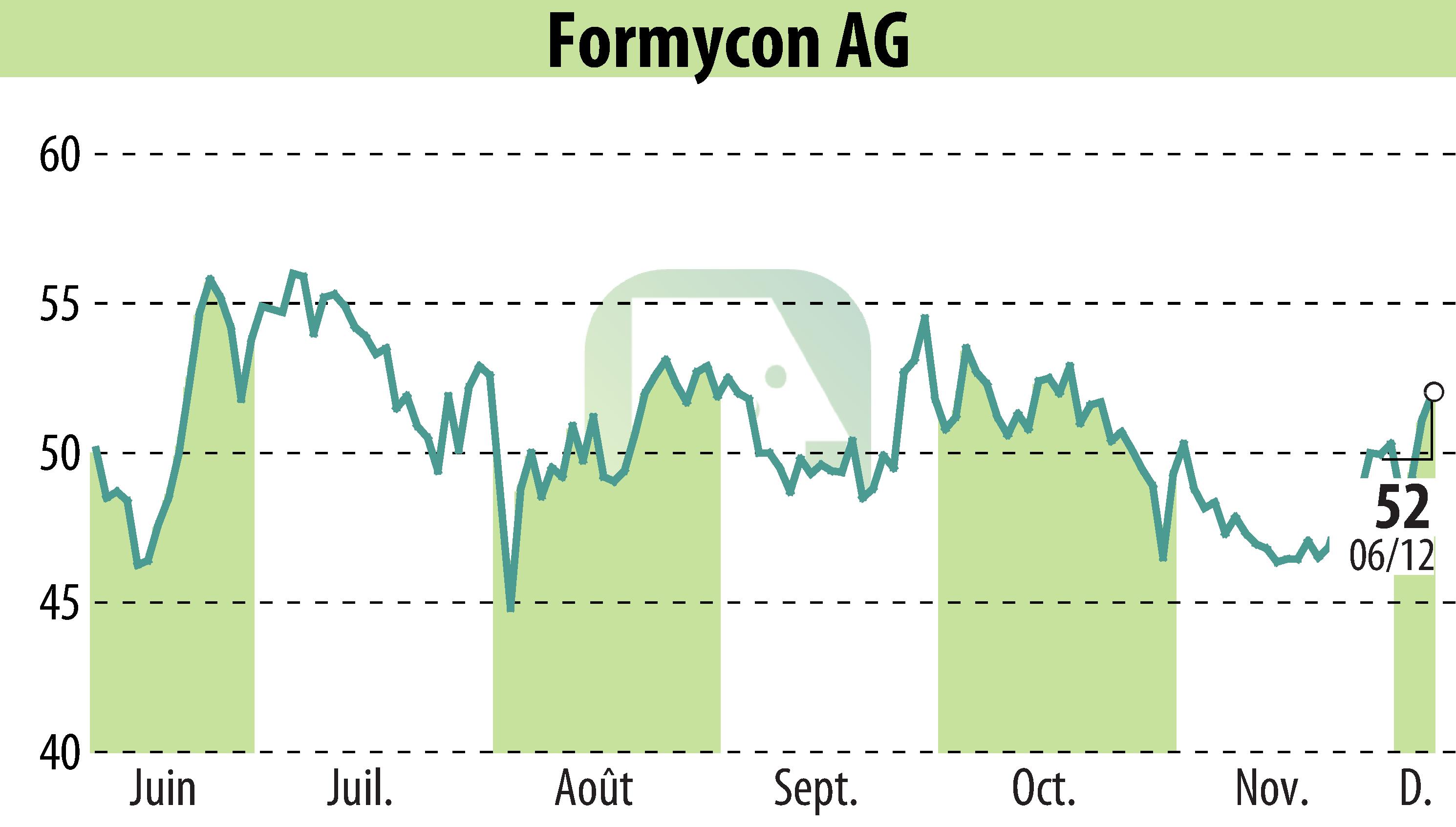
Formycon AG and MS Pharma have announced a licensing and supply agreement for the commercialization of FYB202, a biosimilar to ustekinumab, in the Middle East and North Africa (MENA) region. This agreement allows MS Pharma to license, produce, and commercialize FYB202 in the region, particularly at a new biosimilars site in Saudi Arabia.
Formycon's previous agreements include a global market license with Fresenius Kabi. The partnership with MS Pharma is expected to boost the uptake of FYB202 as a treatment for chronic inflammatory diseases. MS Pharma's expansion includes local production of biosimilars in Saudi Arabia, aligning with the country's biotech strategy.
The U.S. FDA and European Commission granted approval for FYB202 in September 2024, and MS Pharma will soon seek regulatory approval in MENA countries.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
