on NANOBIOTIX (EPA:NANO)
Nanobiotix successfully completes phase 1 study in pancreatic cancer
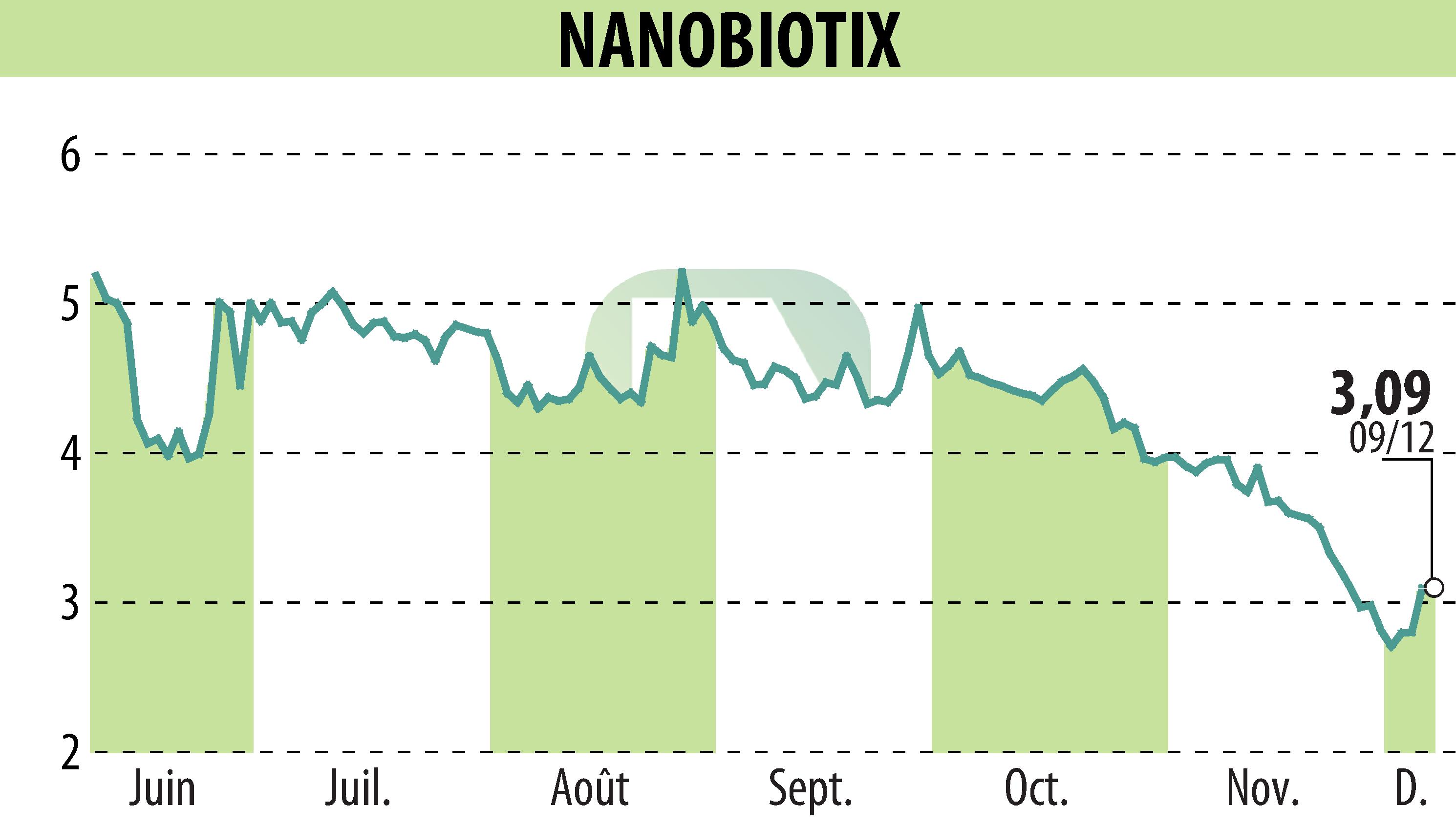
Nanobiotix announced the completion of its Phase 1 study of NBTXR3 (JNJ-1900) in pancreatic cancer. Results showed a median survival from diagnosis of 23 months for 22 patients, surpassing historical data of 19.2 months from MD Anderson Cancer Center.
The study demonstrated a favorable safety profile for NBTXR3 activated by radiation therapy. As a result, the FDA approved an amended protocol for a new cohort combining NBTXR3 with standard chemotherapy, with enrollment ongoing.
The full results of the study will be presented at a medical conference in the first half of 2025. Nanobiotix hopes that this innovation can bring significant benefits to patients.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NANOBIOTIX news
