on ORPHAN SYNERGY EUROPE-PHARMA (EPA:OSE)
OSE Immunotherapeutics Launches Phase 3 Study for Tedopi® in Lung Cancer
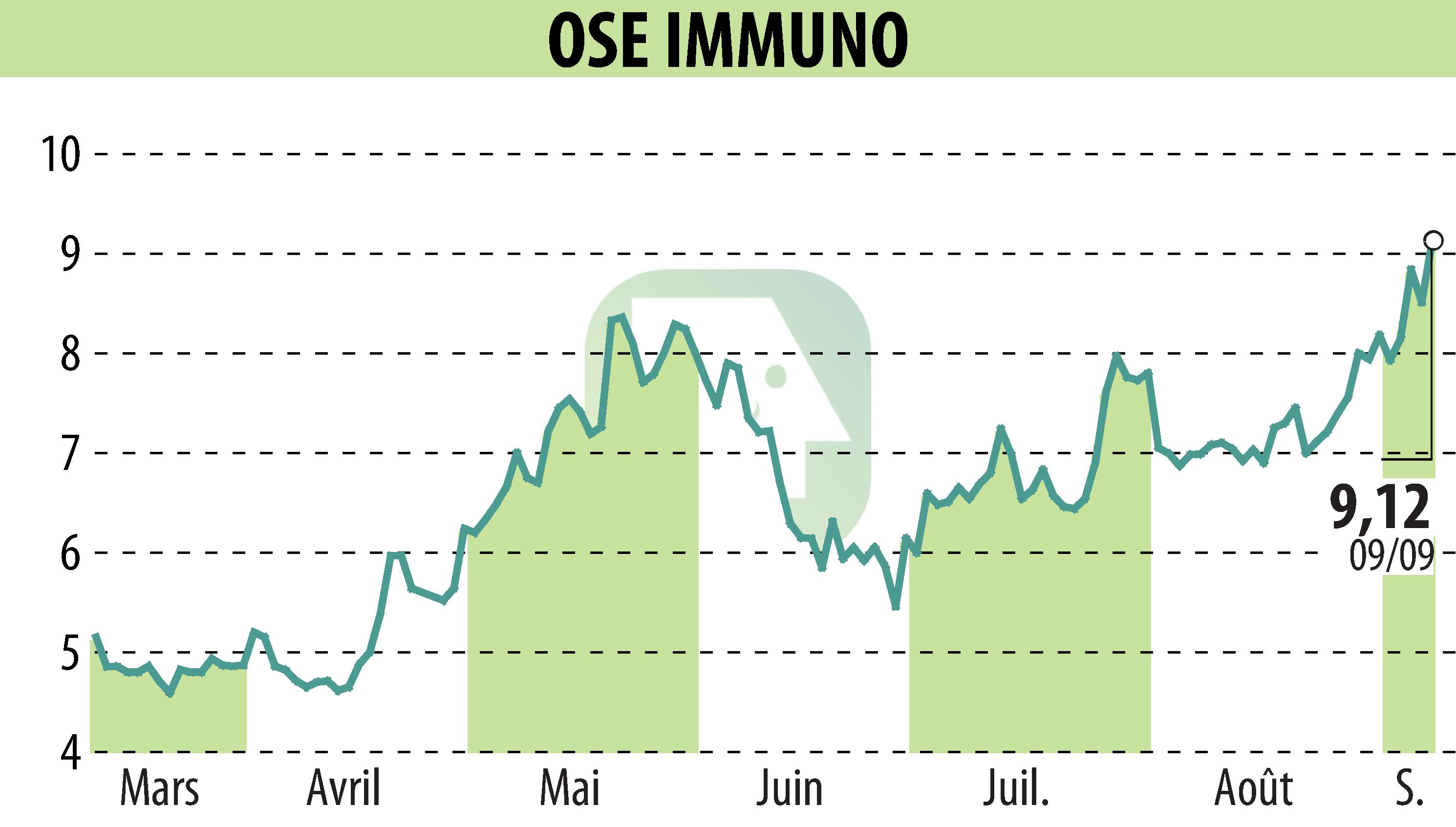
OSE Immunotherapeutics has initiated a global Phase 3 clinical trial for its cancer vaccine, Tedopi®, targeting metastatic non-small cell lung cancer (NSCLC) patients with secondary resistance to immune checkpoint inhibitors (ICI).
The trial, named Artemia, has commenced following regulatory approvals in 14 countries, including the United States, Canada, and Europe. The study compares Tedopi® monotherapy against standard treatments, aiming to improve overall survival in HLA-A2 positive patients.
This international trial will enroll 363 patients. Early presentations have taken place at the World Conference on Lung Cancer and are scheduled for the European Society for Medical Oncology congress.
Dr. Stephen Liu, a key investigator, expressed optimism about the trial, highlighting Tedopi®'s potential to offer a safer alternative to chemotherapy by utilizing patients' immune systems.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ORPHAN SYNERGY EUROPE-PHARMA news
