on ORPHAN SYNERGY EUROPE-PHARMA (EPA:OSE)
OSE Immunotherapeutics Reveals Promising Phase 2 Results for Ulcerative Colitis Treatment
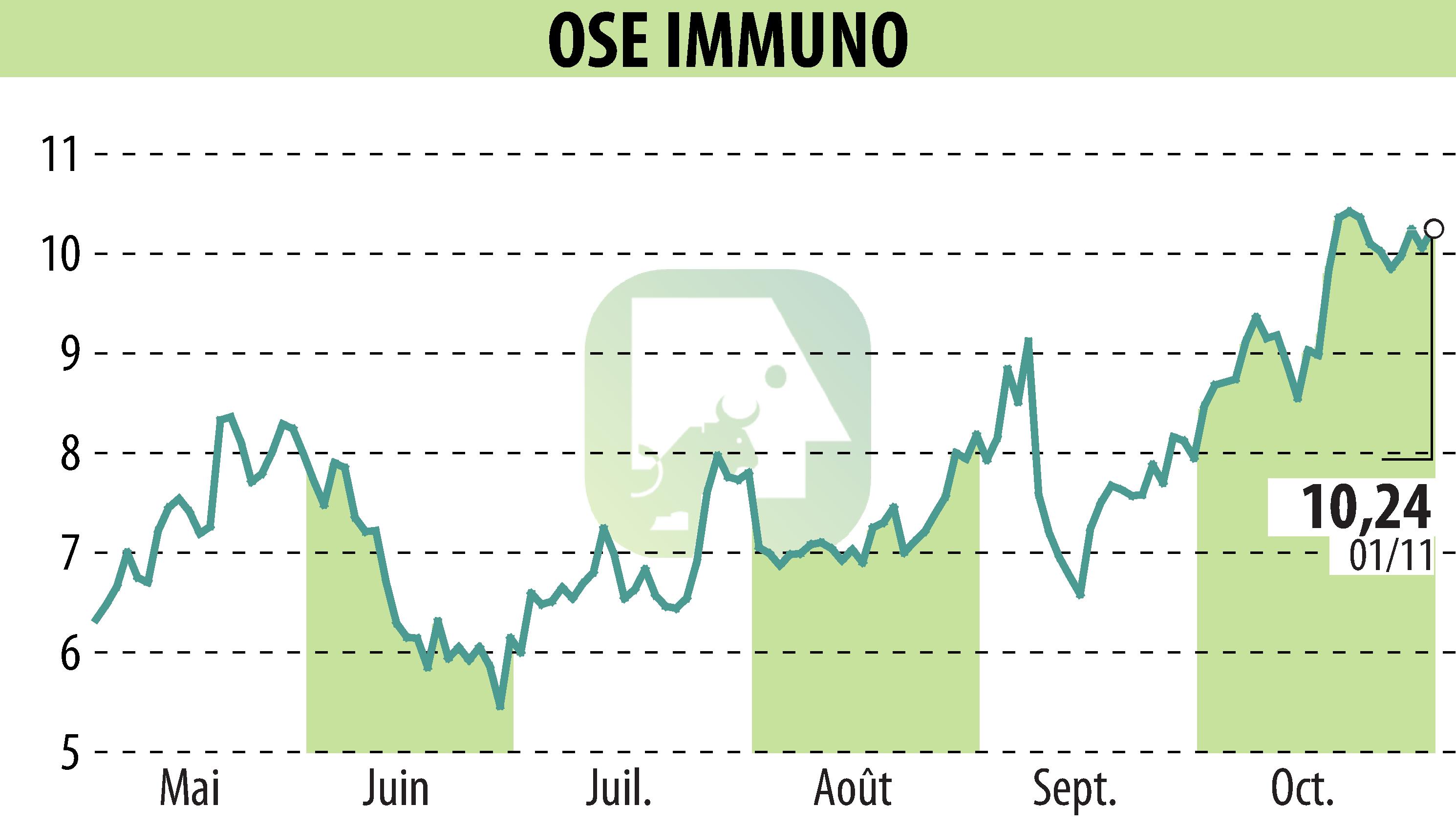
OSE Immunotherapeutics has announced favorable results from its Phase 2 CoTikiS study of Lusvertikimab for ulcerative colitis. The study, which involved 136 participants, revealed that Lusvertikimab significantly improved the Modified Mayo Score compared to placebo, achieving the trial's primary endpoint. Notably, both tested doses, 450mg and 850mg, demonstrated statistically significant improvements.
Beyond the primary endpoint, Lusvertikimab showed strong results in secondary measures, including clinical and endoscopic remission. The safety profile was consistently positive, with no serious adverse events recorded during the 34-week study, combining the 10-week induction and 24-week open-label periods.
The findings posit Lusvertikimab as a potential first-in-class interleukin-7 antagonist for inflammatory and autoimmune conditions, marking a significant milestone in OSE's clinical development strategy.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ORPHAN SYNERGY EUROPE-PHARMA news
