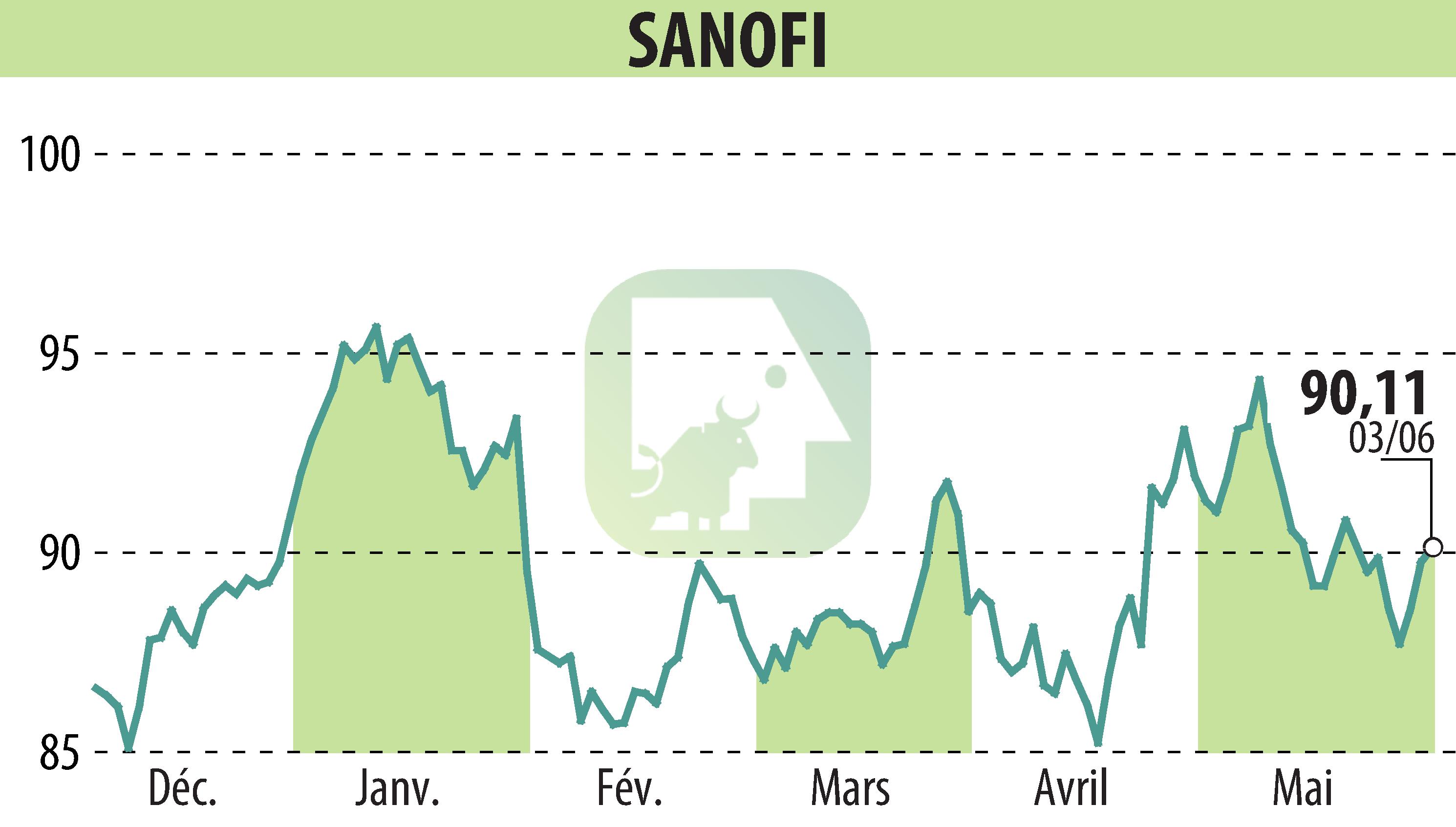
on SANOFI-AVENTIS (EPA:SAN)
Phase III study: Sarclisa improves progression-free survival of patients with multiple myeloma
Sanofi announces promising results for its monoclonal antibody Sarclisa (isatuximab) in combination with a VRd protocol (bortezomib, lenalidomide and dexamethasone). In the Phase III IMROZ study, this treatment reduced the risk of disease progression or death by 40% in patients with newly diagnosed multiple myeloma who were not eligible for transplantation.
At the data cut-off date, the results show a median progression-free survival not reached for Sarclisa-VRd compared to 54.3 months for VRd alone. Additionally, progression-free survival at 60 months was 63.2% for the Sarclisa-VRd group, compared to 45.2% for the VRd group.
Grade ≥3 adverse events affected 91.6% of patients on Sarclisa-VRd and 84% on VRd. No new security signals have been detected. These results are published in the NEJM and presented at ASCO.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
