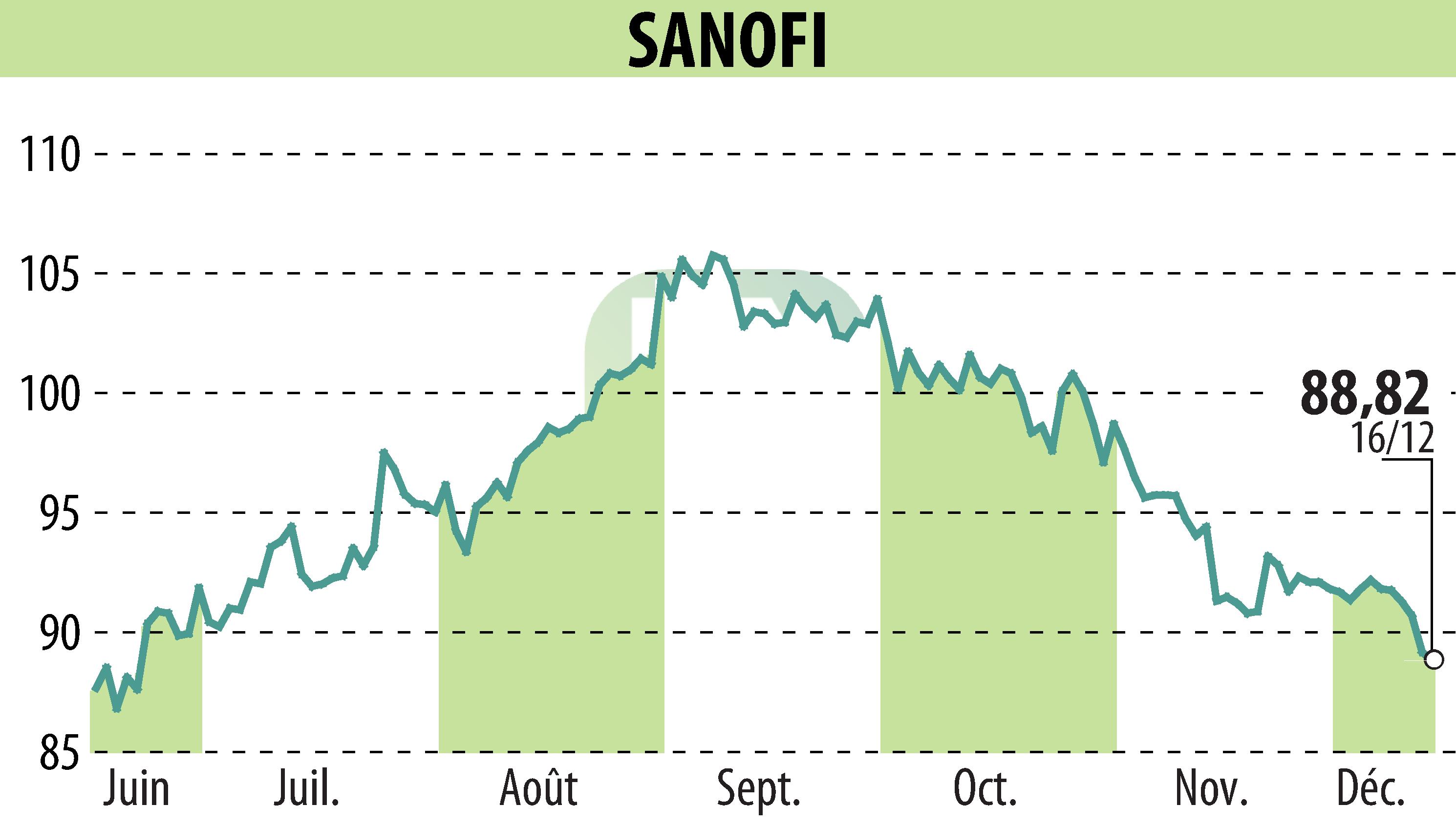
on SANOFI-AVENTIS (EPA:SAN)
Sanofi and Teva Announce Promising Phase 2b Results for Duvakitug
Sanofi and Teva Pharmaceuticals have released encouraging results from their phase 2b RELIEVE UCCD study, indicating the potential of duvakitug, a monoclonal antibody, in treating ulcerative colitis (UC) and Crohn’s disease (CD). The study met its primary endpoints, showing significant clinical remission and endoscopic response in UC and CD patients, respectively.
In UC, 47.8% of high-dose patients achieved clinical remission, starkly higher than the 20.45% on placebo. CD patients similarly benefited, with 47.8% reaching an endoscopic response compared to 13% on placebo. The absence of significant safety signals supports the drug’s tolerability.
Pending regulatory discussions, Sanofi and Teva plan to commence phase 3 trials. These results bolster Sanofi's and Teva's commitment to advancing innovative treatments for inflammatory bowel disease.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
