on SANOFI-AVENTIS (EPA:SAN)
AlphaMedixTM: Positive Phase 2 Results for the Treatment of GEP-NETs
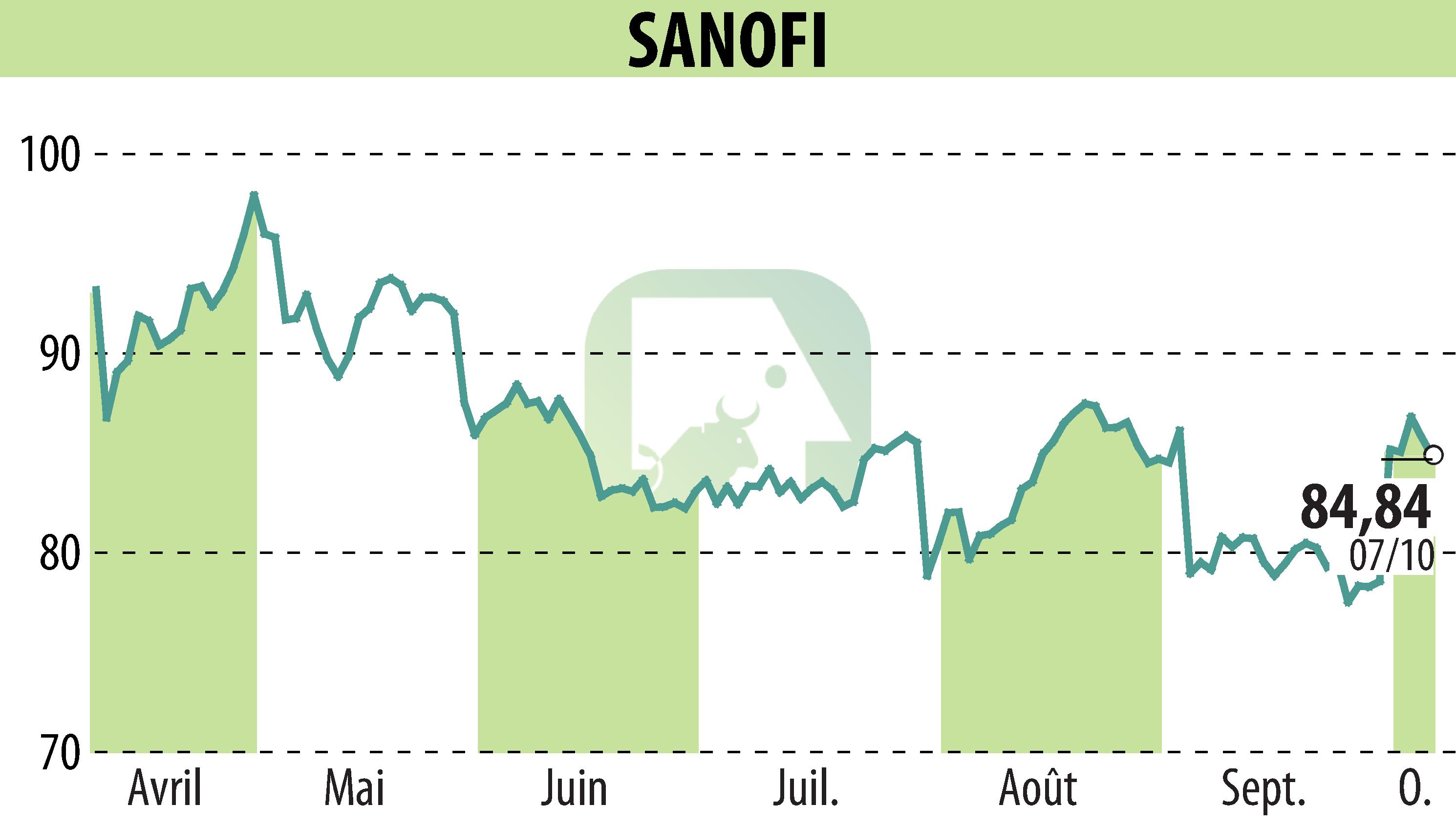
Sanofi-Avantis and Orano Med announced that their investigational therapy AlphaMedixTM (212Pb-DOTAMTATE) has met primary efficacy endpoints in a Phase 2 clinical trial. This trial aims to evaluate its impact on difficult-to-treat gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
The results showed clinically significant benefits in terms of progression-free survival and overall survival, in addition to an acceptable safety profile. This innovative therapy targets somatostatin receptors, which are present in the majority of NETs.
In February 2024, AlphaMedixTM had already received Breakthrough Therapy Designation from the FDA, highlighting its promising potential. Discussions with health authorities will continue to quickly make this therapeutic option available to patients. The full results will be presented at the ESMO 2025 congress.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
